Preparation method of high-purity dasatinib and by-product of dasatinib
A dasatinib, high-purity technology, applied in the field of preparation of dasatinib, can solve problems such as difficulty in separation and impact on the quality of the final product of dasatinib, and achieve the effect of small side effects, high safety and definite curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Example 1 Preparation of compound 6
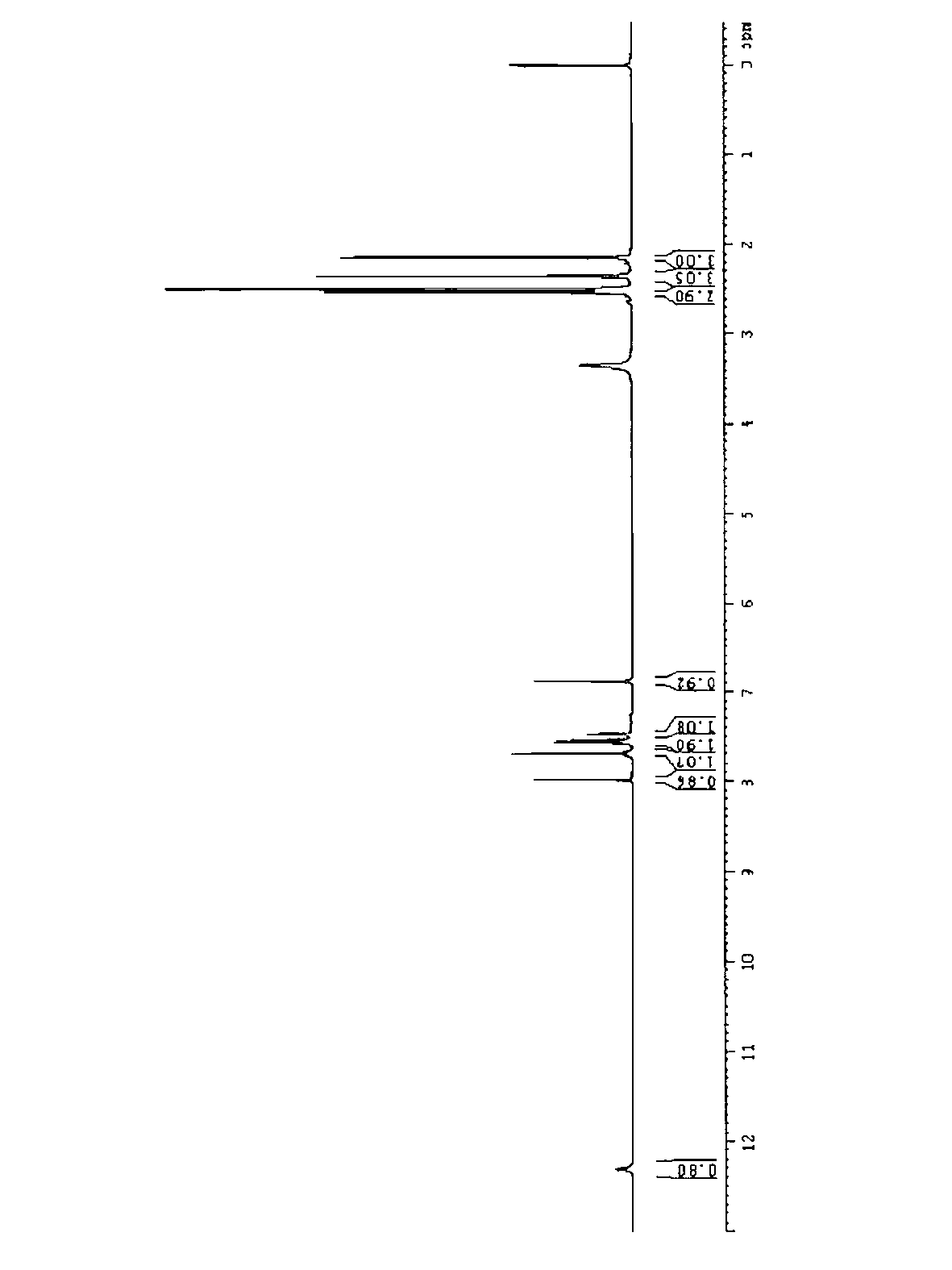
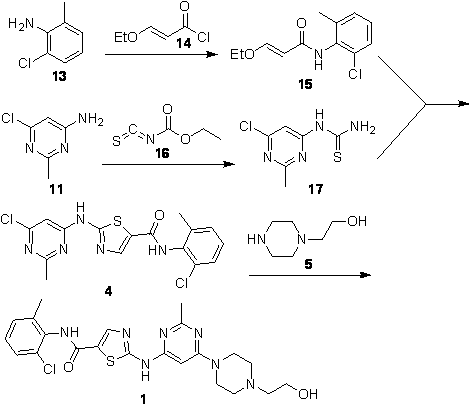
[0066] Add 300ml of tetrahydrofuran and 7g of potassium hydride into a 500ml three-necked flask, cool in an ice-water bath, add 2-amino-N-(2-chloro-6-methylphenyl)-5-thiazolecarboxamide (20g, 74.7mmol), React for 1 hour. Add 4,6-dichloro-2-methylpyrimidine (25 g, 153.3 mmol), heat to reflux, monitor by HPLC, and react for 4 hours. Cool in an ice-water bath, quench with 2N hydrochloric acid, filter, and spin dry. The obtained solid was purified by column, and the eluent was ethyl acetate:petroleum ether=1:3. Obtain compound 6 product 22 grams, its NMR H spectrum sees attached figure 1 .
[0067]
Embodiment 2
[0068] Example 2 Preparation of Compound 6
[0069] Add 300ml of dioxane and 8.9 grams of sodium hydride into a 500ml three-necked flask, cool in an ice-water bath, add 2-amino-N-(2-chloro-6-methylphenyl)-5-thiazolecarboxamide (20g, 74.7 mmol), reacted for 2 hours. Add 4,6-dichloro-2-methylpyrimidine (36 g, 220.9 mmol), heat to reflux, monitor by HPLC, and react for 6 hours. Cool in an ice-water bath, quench with 2N hydrochloric acid, filter, and spin dry. The obtained solid was purified by column, and the eluent was ethyl acetate:petroleum ether=1:3. 19.3 g of compound 6 was obtained.
[0070]
Embodiment 3
[0071] Example 3 Preparation of Compound 6
[0072] Add 500ml of ethylene glycol dimethyl ether, 5g of sodium ethoxide and 2g of sodium hydride into a 500ml three-necked bottle, cool in an ice-water bath, add 2-amino-N-(2-chloro-6-methylphenyl)-5-thiazole Amide (20g, 74.7mmol), reacted for 2 hours. Add 4,6-dichloro-2-methylpyrimidine (60.8g, 373.5mmol), heat to reflux, monitor by HPLC, and react for 5 hours. Cool in an ice-water bath, quench with 2N hydrochloric acid, filter, and spin dry. The obtained solid was purified by column, and the eluent was ethyl acetate:petroleum ether=1:3. 20.1 g of Compound 6 was obtained.
[0073]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com