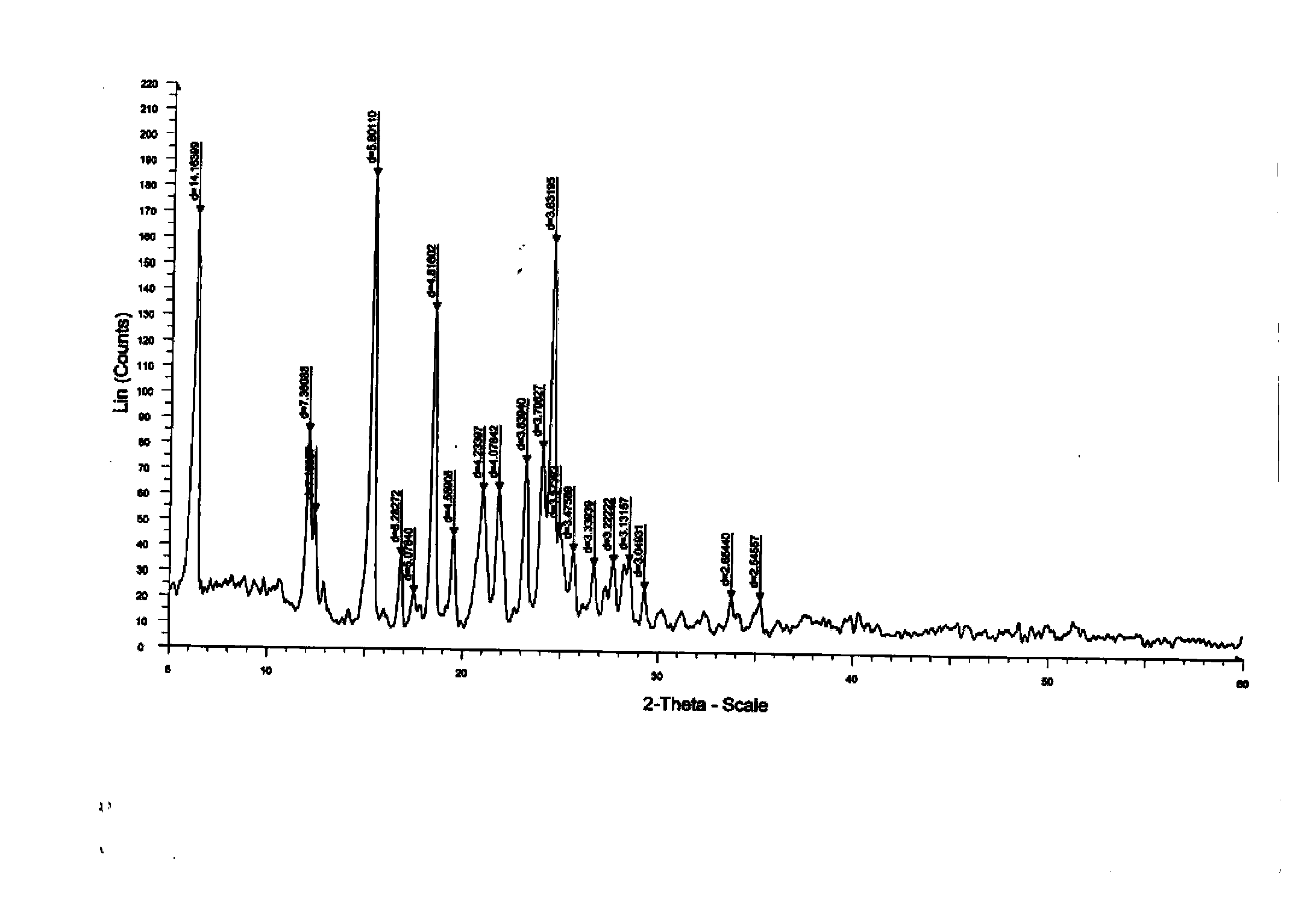
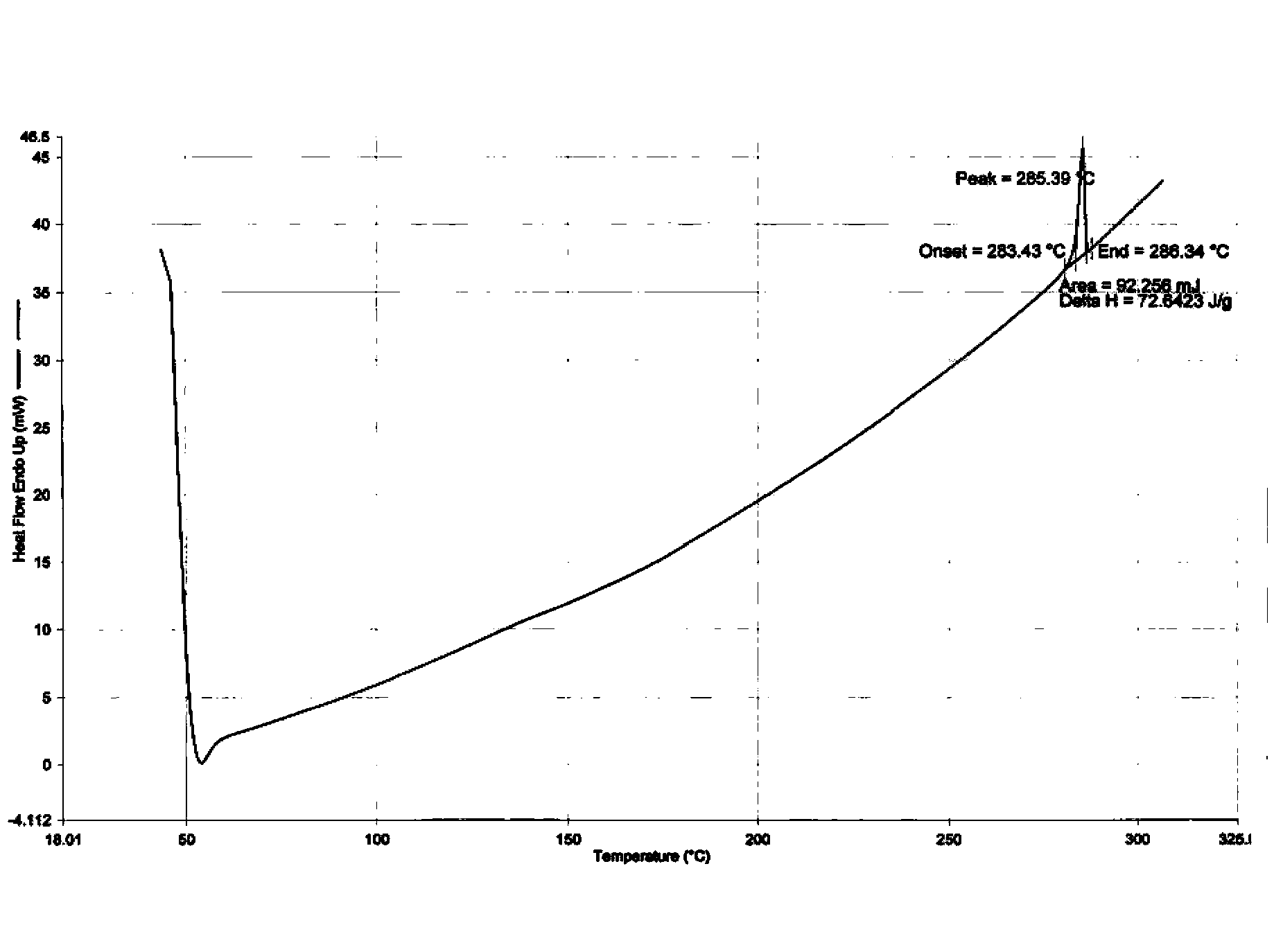
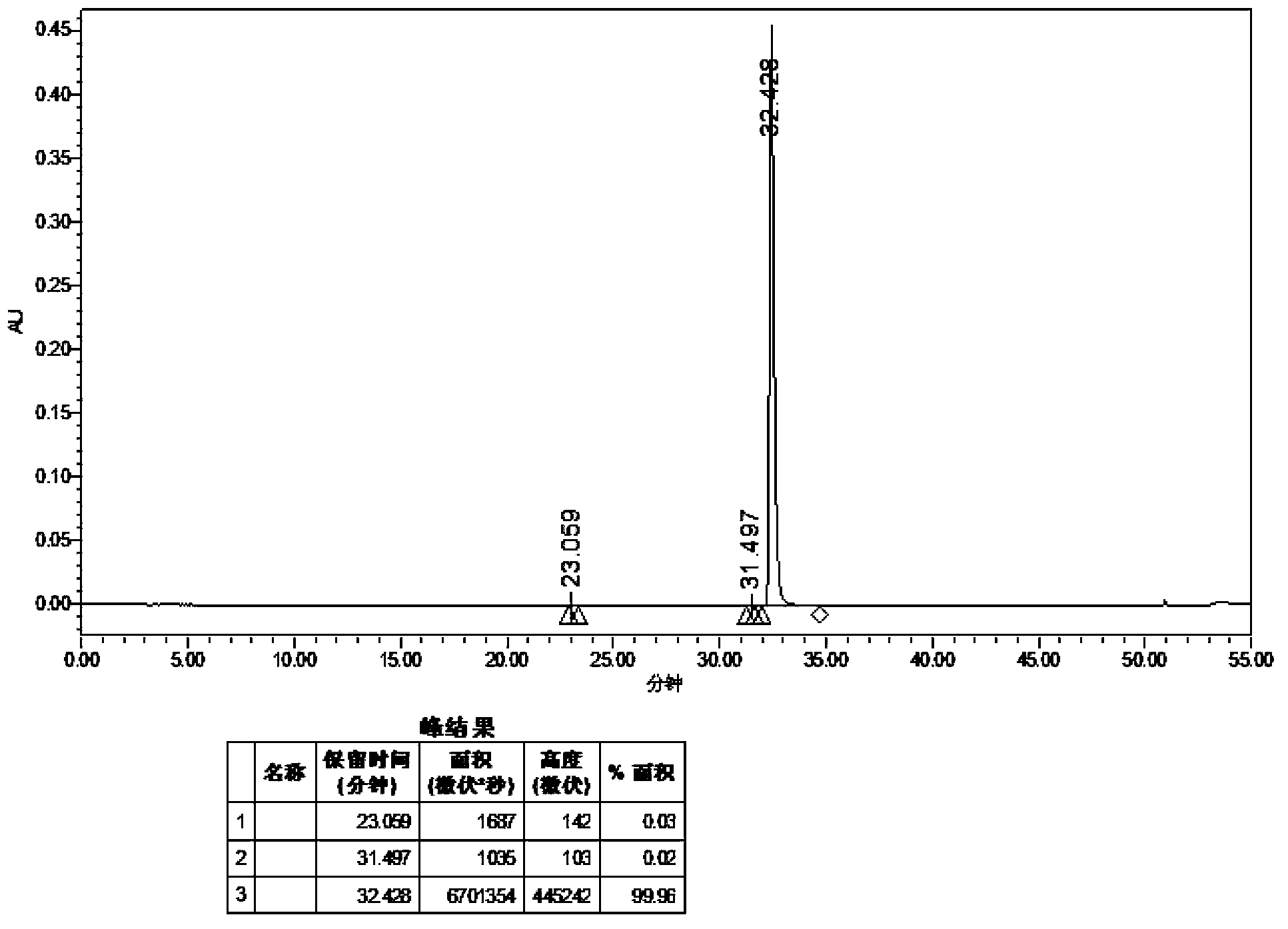
Dasatinib polycrystalline form medicament and preparation method thereof
A dasatinib polymorphic technology, which is applied in the field of dasatinib polymorph III and its preparation, can solve the problems of many temperature control points, unsuitable disposal of waste residue, and great harm to operators, and achieve good fluidity and compressibility, stable and controllable quality, and mild process conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Preparation of type Ⅲ dasatinib polymorph
[0092] Dissolve 2.0 g of dasatinib in 20 mL of dimethyl sulfoxide, stir at 30°C for 1 h, add 120 mL of acetone, stir for 18 h, filter with suction, and dry to obtain 1.7 g of an off-white solid with a yield of 88.5% and a purity of 99.96%.
Embodiment 2
[0094] Preparation of type Ⅲ dasatinib polymorph
[0095] Dissolve 3.0g of dasatinib in 24mL of N,N-dimethylformamide, stir at 70°C for 0.5h, add 48mL of ethyl acetate, stir for 10h, filter with suction, and dry to obtain 2.61g of off-white solid, yield 87% , 99.78% purity.
Embodiment 3
[0097] Preparation of type Ⅲ dasatinib polymorph
[0098] Dissolve 2.0g of dasatinib in 4mL of N,N-dimethylacetamide, stir at 30°C for 0.5h, add 8mL of ethyl acetate, stir for 8h, filter with suction, and dry to obtain 1.76g of off-white solid, yield 88% , with a purity of 99.74%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| angle of repose | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com