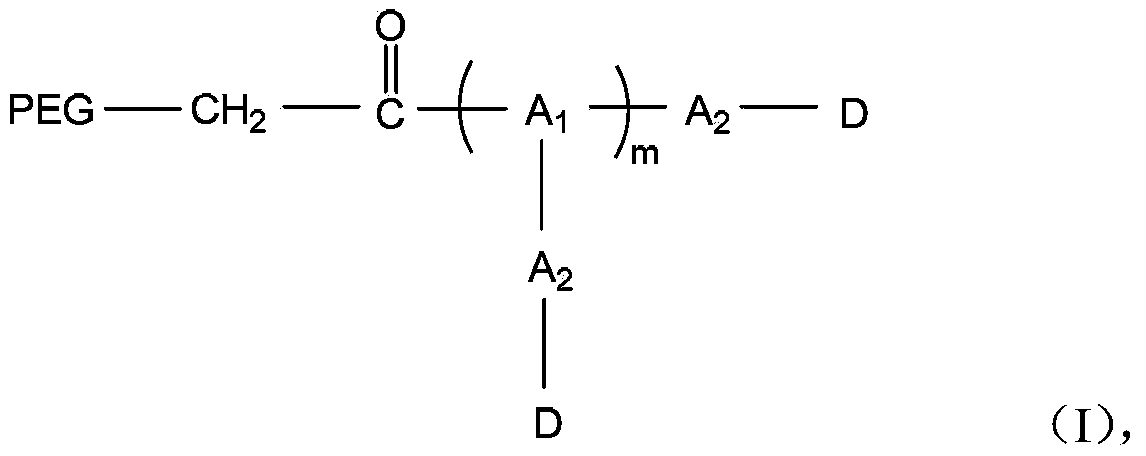
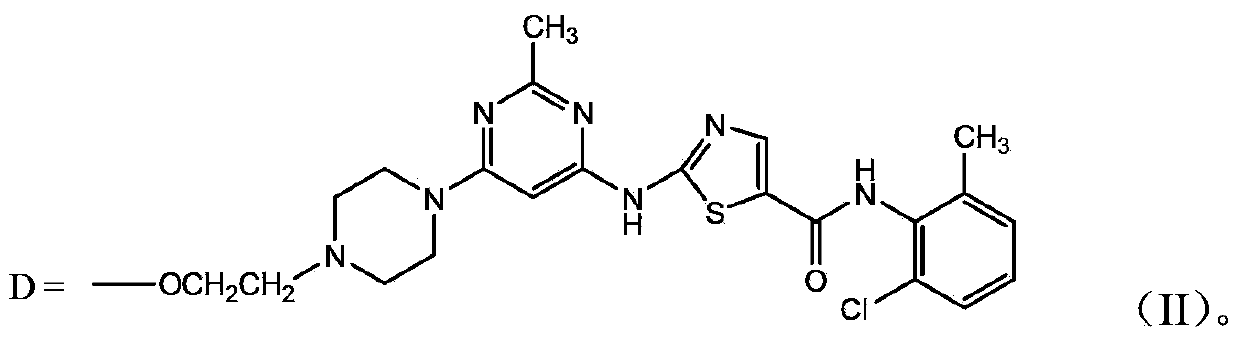
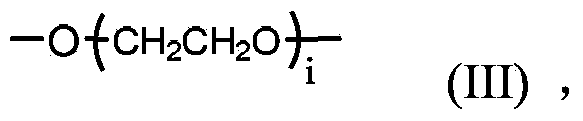Polyethylene glycol-amino acid oligopeptide-dasatinib conjugate and pharmaceutical composition thereof
A technology of polyethylene glycol and polyethylene glycol, applied in the direction of drug combination, antineoplastic drugs, pharmaceutical formulations, etc., to achieve the effect of increasing the load rate and improving the clinical effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Preparation of monomethoxypolyethylene glycol (number average molecular weight 20000)-amino acid pentapeptide-dasatinib conjugate (DSR-1)
[0055]
[0056] 29.4g (0.2mol) of L-(+)-glutamic acid, 40g (0.23mol) of p-toluenesulfonic acid, and 80mL of benzyl alcohol were dissolved in 500mL of toluene, and 11mL of water was separated by reflux under nitrogen protection. 150mL. Cool to 50°C, pour the reaction solution into a beaker filled with 600mL petroleum ether, stir for 1h, and collect the precipitate by filtration. After the filter cake was heated and dissolved with 280 mL of 95% ethanol, the heating was stopped and cooled overnight. The precipitate was collected by filtration and dried in vacuo to obtain 61 g of L-(+)-dibenzyl glutamate p-toluenesulfonate.
[0057] Dissolve 30g (0.06mol) of dibenzyl glutamate p-toluenesulfonate in 500mL of dichloromethane, add 20.86g (0.062mol) of tert-butoxycarbonyl-L-glutamic acid-5-benzyl ester, DMAP7.55g (0.062mol), HOBt8.35g...
Embodiment 2
[0063] Preparation of Y-type polyethylene glycol (number average molecular weight 30000)-amino acid pentapeptide-dasatinib conjugate (DSR-2)
[0064]
[0065] N-tert-butoxycarbonyl glutamate benzyl ester dipeptide (Boc-Glu(obzl)-Glu(obzl)-obzl) 0.78g (Example 1) was dissolved in 7mL of dichloromethane, added 3mL of trifluoroacetic acid, room temperature Reaction 2h. Remove the solvent, add 100mL of dichloromethane, adjust pH=7-8 with 5% sodium bicarbonate solution. Extraction and liquid separation, the organic phase was washed twice with 5% sodium bicarbonate solution, and dried over anhydrous sodium sulfate. After filtration, the filtrate was directly added to the reaction flask, and under nitrogen protection, 40.0 g of Y-polyethylene glycol acetic acid (30K), 245 mg (2 mmol) of DMAP, and 135 mg (1 mmol) of HOBt were added, and after all were dissolved, 412 mg (2 mmol) of DCC was added. The reaction was stirred overnight at room temperature. Filter, remove the solvent b...
Embodiment 3
[0067] Preparation of monomethoxy polyethylene glycol (number average molecular weight 40000)-amino acid heptapeptide-dasatinib conjugate (DSR-3)
[0068]
[0069] 6.47 g (0.01 mol) of benzyl N-tert-butoxycarbonyl glutamate dipeptide (Example 1) was dissolved in 15 mL of dichloromethane, 6 mL of trifluoroacetic acid was added, and reacted at room temperature for 2 h. Remove the solvent, add 100mL of dichloromethane, adjust pH=7-8 with 5% sodium bicarbonate solution. Extraction and liquid separation, the organic phase was washed twice with 5% sodium bicarbonate solution, and dried over anhydrous sodium sulfate. After filtration, the filtrate was directly added to the reaction flask, under the protection of nitrogen, 3.37g (0.01mol) of tert-butoxycarbonyl-L-glutamic acid-5-benzyl ester, 1.22g (0.01mol) of DMAP and 1.35g (0.01mol) of HOBt were added. mol), DCC2.39g (0.011mol) dichloromethane solution was added dropwise after complete dissolution. After dropping, the closed sys...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mean tumor volume | aaaaa | aaaaa |
| Mean tumor volume | aaaaa | aaaaa |
| Relative tumor proliferation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com