Novel anelgesic combination
a technology of anelgesics and combination, applied in the direction of dragees, coatings, pharmaceutical delivery mechanisms, etc., can solve the problems of tramadol may produce certain side effects, still has certain commonly reported side effects, and may have serious upper gastrointestinal complications. achieve the effect of reducing the pain scor
Inactive Publication Date: 2008-02-07
NECTID INC
View PDF23 Cites 55 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
[0037] One object of the present invention is to provide methods, which can effectively be used in the treatment of pain and pain related diseases wherein the methods comprise administration of a therapeutically effective amount of an NSAID such as naproxen and administration of a therapeutically effective amount of a slow release Tramadol to a patient in need thereof.
[0053] The term “candidate for sustained release” encompasses all the characteristics of a drug which make it a candidate for formulating it into an extended release fashion like a short elimination half life and consequent dosing of more than once a day, a single dose product given in an extended fashion to achieve better clinical results and avoid side effects associated with an immediate release etc
Problems solved by technology
However, it still has certain commonly reported side effects include nausea, constipation, dizziness, headache, drowsiness, and vomiting.
Most anti-inflammatory drugs have been associated with an increased risk of serious upper gastrointestinal complications.
When given at a dose of 50 mg by rapid i.v. injection, tramadol may produce certain side effects unique to tramadol including hot flushes and sweating.
They, however, produce undesirable side effects and as a result cannot always be given repeatedly or at high doses.
In addition, ibuprofen, aspirin and some other NSAIDs may cause gastrointestinal side effects especially if used repeatedly.
The prior art, however, does not disclose a pharmaceutical composition comprising a slow release tramadol and an NSAID for treating a patient in need there of.
Further prior art doesn't disclose a method of treating pain or pain related disorder comprising a method of administering to a mammal in need thereof, a pharmaceutical composition comprising a slow release tramadol and an NSAID
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
example 2
Treatment E Example 2
[0133]
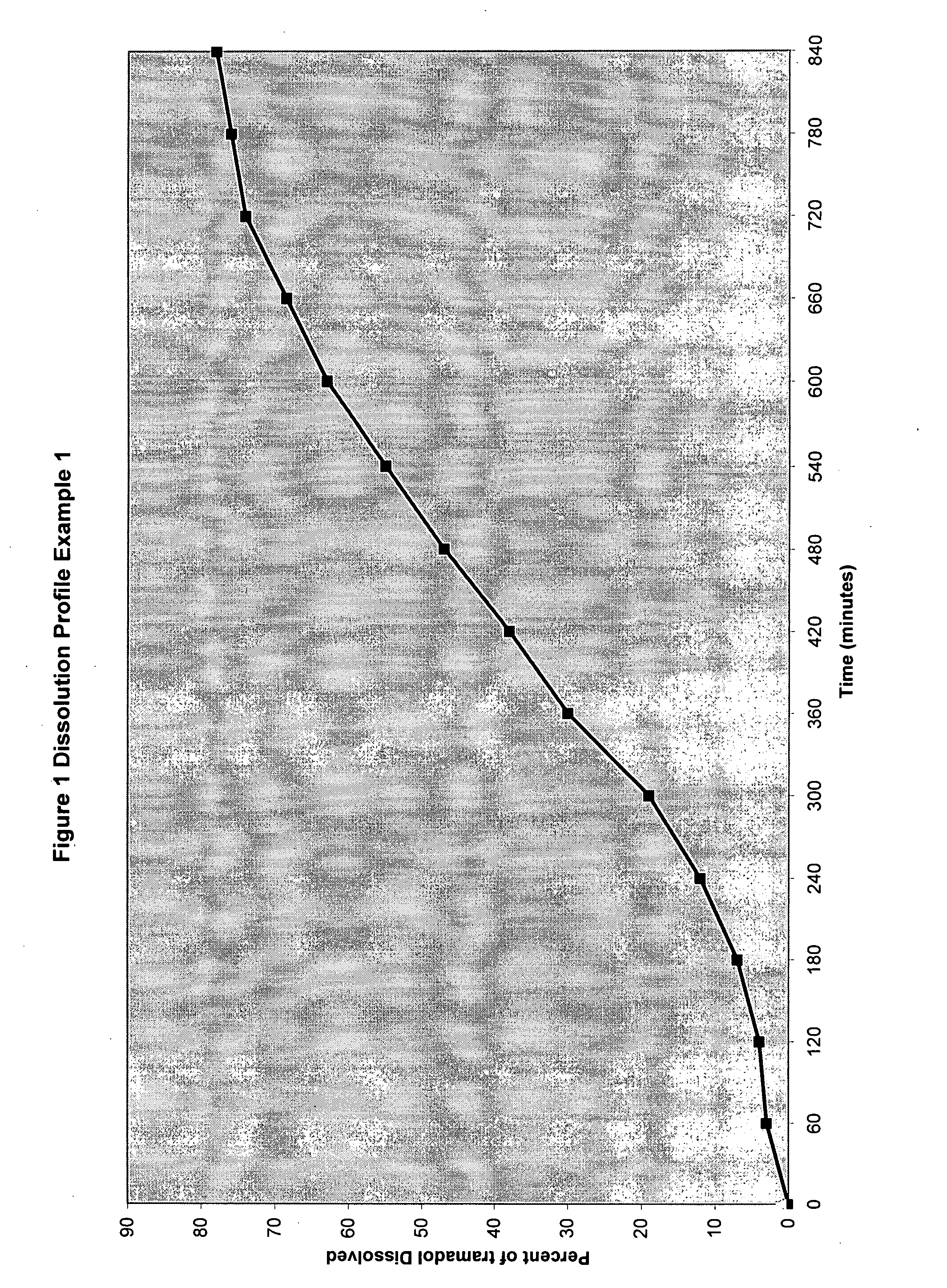
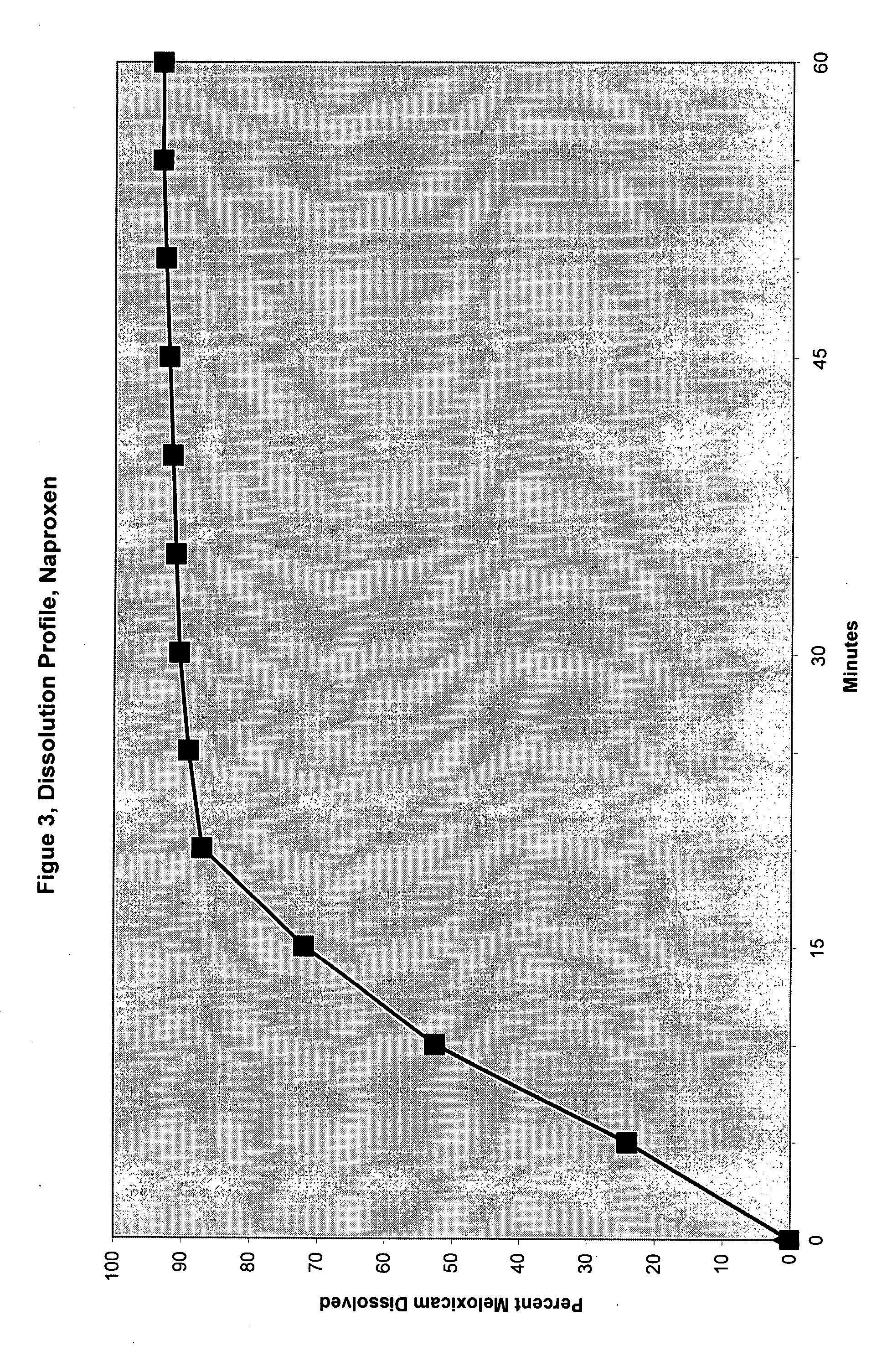
TABLE 6Dissolution Study ConditionsApparatus:USP basket of 10 MeshMedium of dissolution0.1 N HydrochlorideVessel Volume900 mlTemperature37′-38″ C.Wavelength271 nmFlow Cell Measurement1 CMSpeed75 RPMRun time900 minutesIntervel for sampling30 Minutes
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| pharmaceutical composition | aaaaa | aaaaa |
| hydrophilic | aaaaa | aaaaa |
| hydrophobic | aaaaa | aaaaa |
Login to View More
Abstract
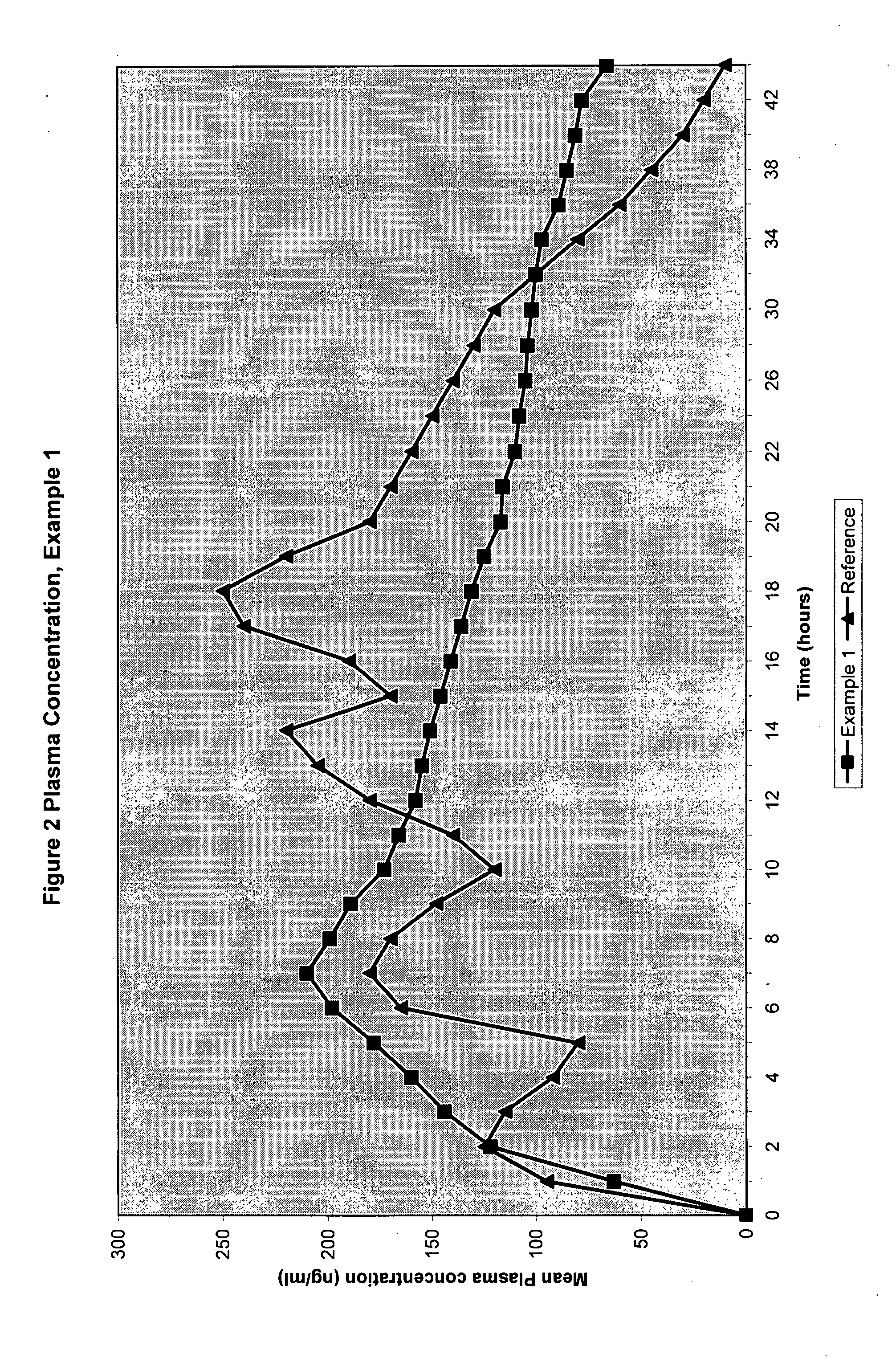
The invention discloses a method of administering a pharmaceutical combination comprising an NSAID and a slow release tramadol to a mammal in need of thereof. This invention further discloses an analgesic combination comprising an NSAID and a slow release tramadol for treating pain and pain related conditions.
Description
FIELD OF THE INVENTION [0001] The present invention provides a method of treating pain and pain related conditions by administering to a patient in need thereof, a therapeutically effective amount of an NSAID and a slow release tramadol combination. The present invention further a pharmaceutical composition comprises of a slow release Tramadol and an immediate release NSAID such as Naproxen BACKGROUND OF THE INVENTION [0002] Tramadol (FORMULA 1) is a centrally acting synthetic opioid analgesic. It is chemically (±) cis-2-[(dimethylamino) methyl]-1-(3-methoxyphenyl)cyclo-hexanol hydrochloride. It is commercially available in form of its hydrochloride salt (Formula II) as Ultram tablets. Tramadol is indicated in the treatment of the management of moderate to moderately severe pain in adults. [0003] Tramadol is not an NSAID and doesn't have the increased risk of stomach ulceration and internal bleeding associated with non-steroidal anti inflammatory drugs (NSAID). However, it still ha...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(United States)
IPC IPC(8): A61K9/24
CPCA61K9/2027A61K9/2866A61K9/209
Inventor SESHA, RAMESH
Owner NECTID INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com