Immune effector cell cryoprotectant and application thereof
A cryopreservation, cell technology, applied in the biological field, can solve the problems of lack of quality, low cell viability, slow proliferation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0091] The preparation method of the cell cryopreservation liquid of the present invention includes the steps: mixing the components of the cell cryopreservation liquid to obtain the cell cryopreservation liquid.
[0092] Cell mixture
[0093] The cell mixture of the present invention includes the following components:
[0094] Cells, and
[0095] The cell cryopreservation solution of the present invention;
[0096] In the cell mixture, the cells are not particularly limited, as long as they can meet the requirements of cell freezing and resuscitation. Preferably, the cells are immune effector cells. More preferably, the cells include (but are not limited to) one or more selected from the group consisting of CAR-T cells, CIK cells, NK cells and cytotoxic T lymphocytes (CTL). Preferably
[0097] In the cell mixture, the cell cryopreservation solution is further defined as described above.
[0098] Freezing method
[0099] The method for freezing cells of the present invention includes th...
Embodiment 1
[0134] Example 1 Preparation of CAR-T cells
[0135] Take blood samples from donors No. 1 and No. 2 (both donors No. 1 and No. 2 are healthy adults), and isolate PBMC cells by FICOL. After adherence treatment, they are electrotransformed and cultured to the desired cells the amount. The specific steps were performed according to the preparation method disclosed in Antitumoractivity of EGFR-specific CART cells against non-small-cell lung cancer cells in vitro and in mice, Li et al. Cell Death and Disease (2018) 9:177. CAR-T cells prepared from blood samples from patient #1 and donor subject #2 were named CAR-T1 and CAR-T2, respectively.
Embodiment 2
[0136] Example 2 Preparation of Immune Effector Cell Cryopreservation Solution and Cell Cryopreservation
[0137] Preparation of Immune Effector Cell Cryopreservation Solution
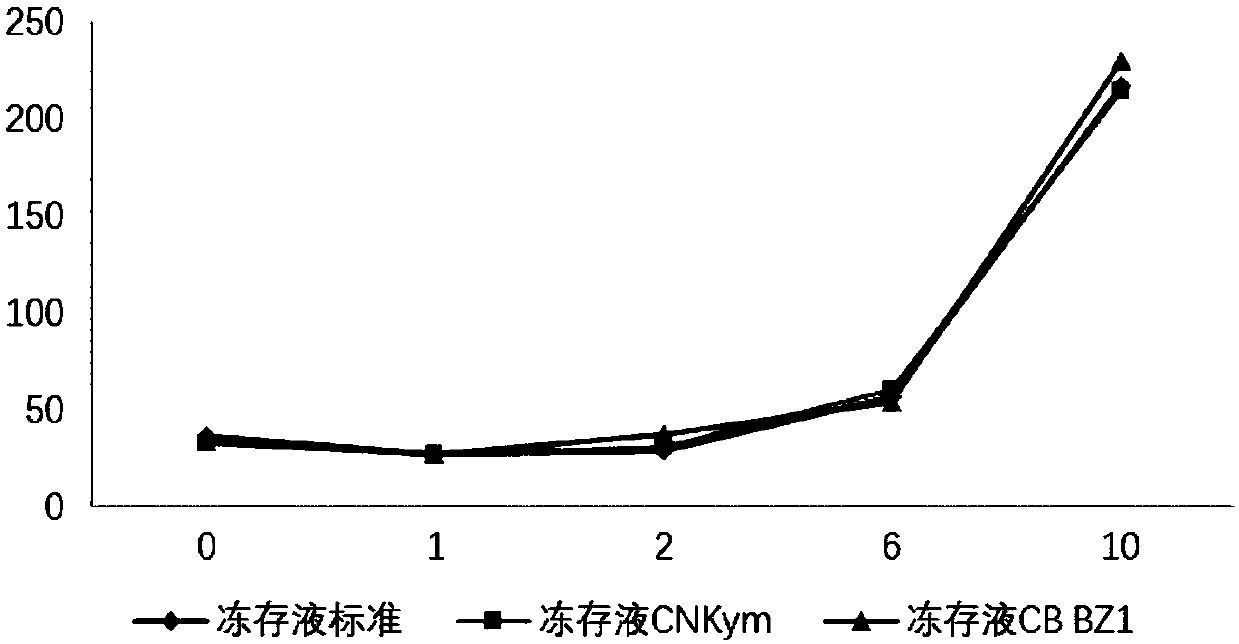
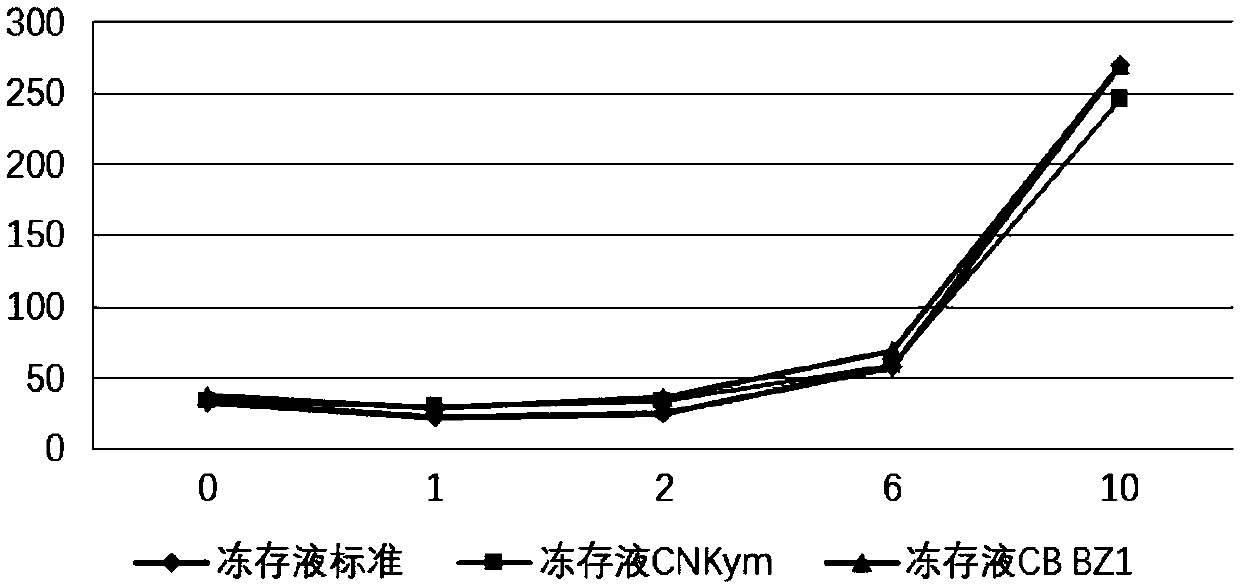
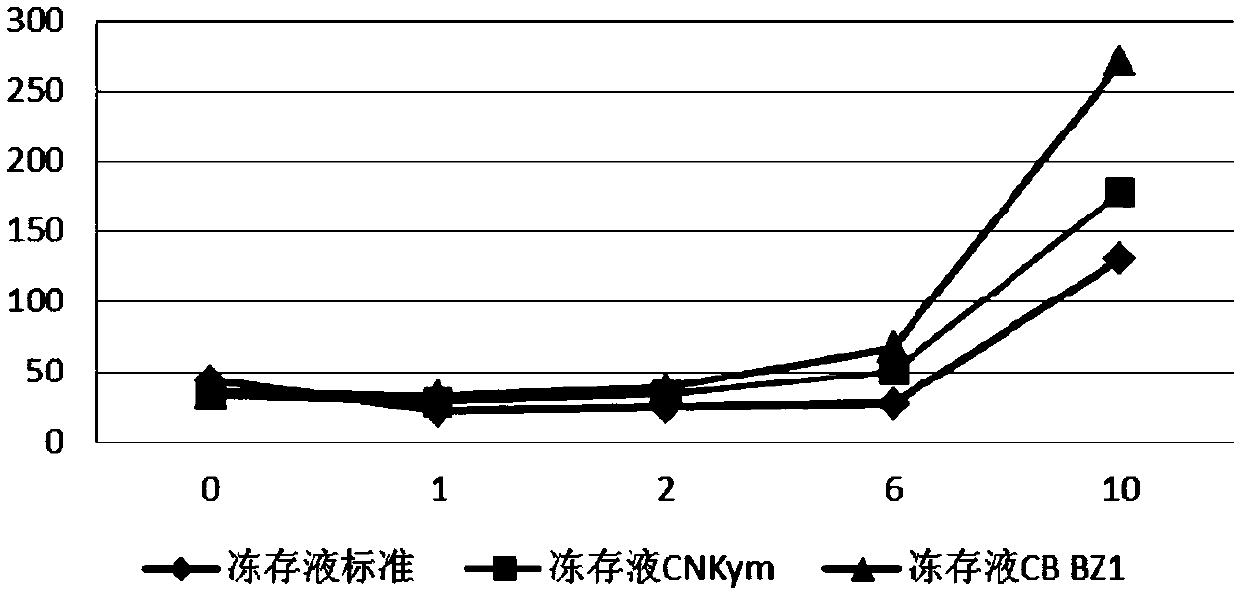
[0138] Mix the ingredients according to the ratio of the cell cryopreservation solution as shown in Table 1, and resuspend the CAR-T1 and CAR-T2 cells prepared in Example 1. The cell density after resuspension is 5x10 7 / ml. The reference cryopreservation fluid standard is the serum-containing cryopreservation fluid routinely used in the field. The reference cryopreservation fluid CNKym is the cryopreservation fluid used by the known Novartis product Kymriah, the cryopreservation fluid CBBZ1, CB BZ2, CB BZ3 and CB BZ4 These are the cryopreservation solutions used in the present invention. The cryopreservation fluid standard and cryopreservation fluid CNKym resuspend CAR-T1 and CAR-T2 cells, CB BZ1 and CB BZ3 respectively resuspend CAR-T1 cells, and CB BZ2 and CB BZ4 cryopreservation fluids resuspend CAR-T2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com