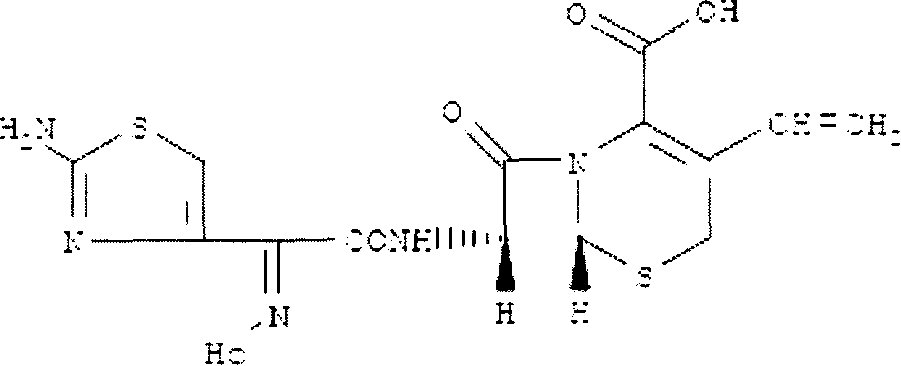
Cefdinir oral disintegration tablet, and its prepn. method
A technology for oral disintegrating tablets and cefdinir, which is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, and pill delivery, etc., can solve the problems of cefdinir oral disintegrating tablets, etc. Ease of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Suspend 25g cefdinir in 7.5g hydroxyethyl cellulose aqueous solution to form solution A, then mix and dissolve 2.5g gelatin, 0.5g spices, 64.5g mannitol and 25g sucrose to form solution B, add the formed solution A to Solution B is mixed, diluted with an appropriate amount of water, mixed well, added to the mold and frozen at low temperature, put into a freeze dryer to vacuumize, sublimated from ice until the material is completely dry, sealed and packaged.
[0064] The total weight of the above-mentioned auxiliary materials is 100g, and 1000 tablets are made, each auxiliary material is 100mg, and the main drug is 25mg of cefdinir, that is, the weight of cefdinir orally disintegrating tablets is 125mg.
Embodiment 2
[0066] Mix the main drug cefdinir 50g with glucose 30g, compressible sorbitol 10g, mannitol 20g, sodium bicarbonate 10g, citric acid 9.9g, saccharin sodium 0.05g, aspartame 0.05, add wetting agent or The binder is made into a soft material, sieved, dried, sized, added with a lubricant, and compressed into 1000 tablets, each weighing 150 mg, containing 50 mg of cefdinir and 100 mg of auxiliary materials.
Embodiment 3
[0068] The main drug cefdinir 100g and carboxymethyl starch sodium 10g, compressible starch 60g, crystal cellulose sodium 30g, sodium lauryl sulfate 3g, mannitol 5g, sodium chloride 1g, flavor 0.5g, 0.5 g of spartame was mixed evenly, and directly compressed into tablets after adding a lubricant to make 1000 tablets, each weighing 210 mg, containing 100 mg of cefdinir and 110 mg of auxiliary materials.
[0069] In the above three embodiments, cefdinir 25mg, 50mg, and 100mg are in three specifications altogether, and when preparing this product, the weight of each tablet is variable. When preparing other orally disintegrating tablets containing cefdinir 10-200mg / tablet, the method is the same as above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com