Crystalline acid salts of cefdinir and process for preparing cefdinir using same
A technology of cefdinir and crystalline acid, which is applied in the field of preparing cefdinir and can solve the problems of low purity and poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
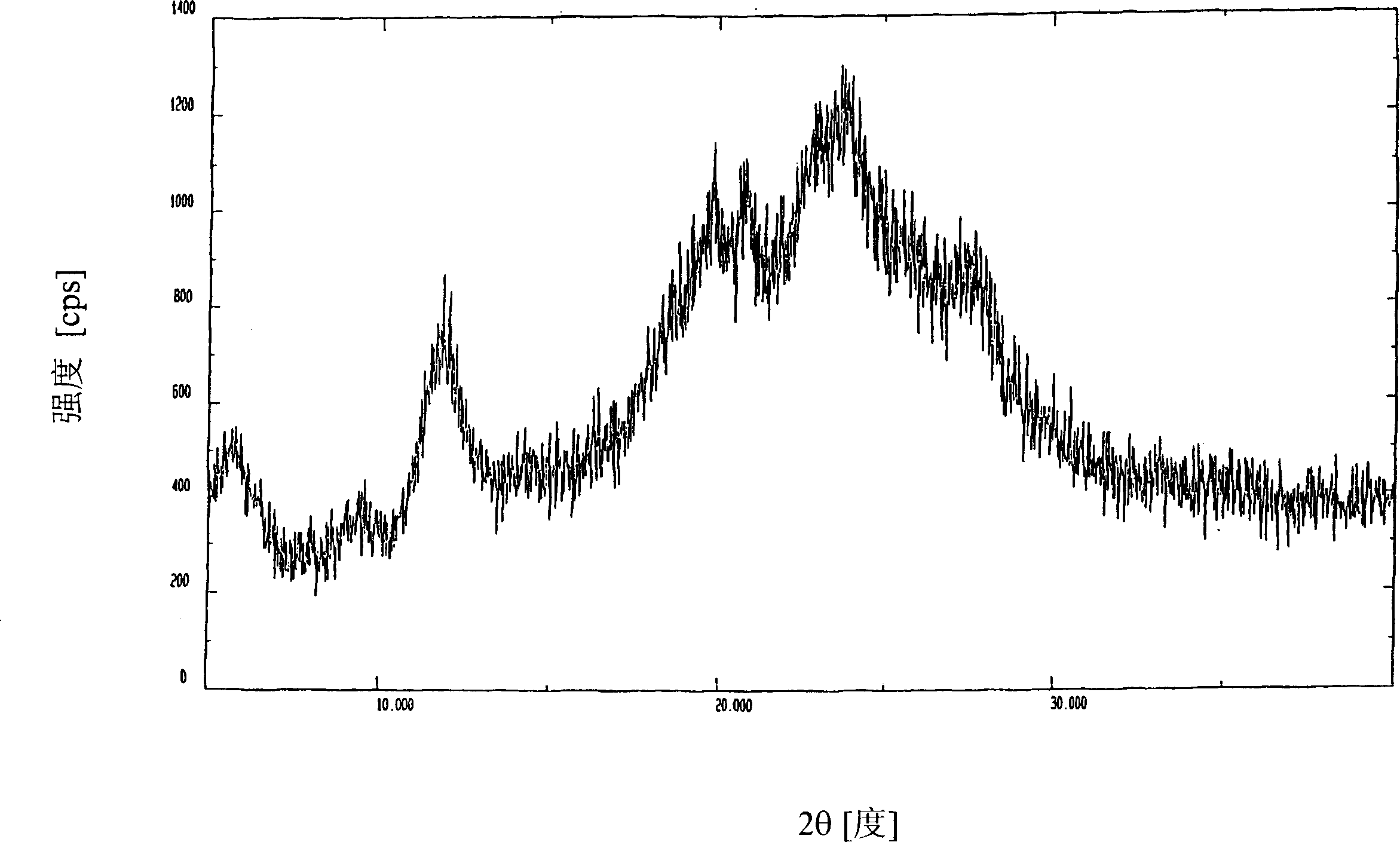
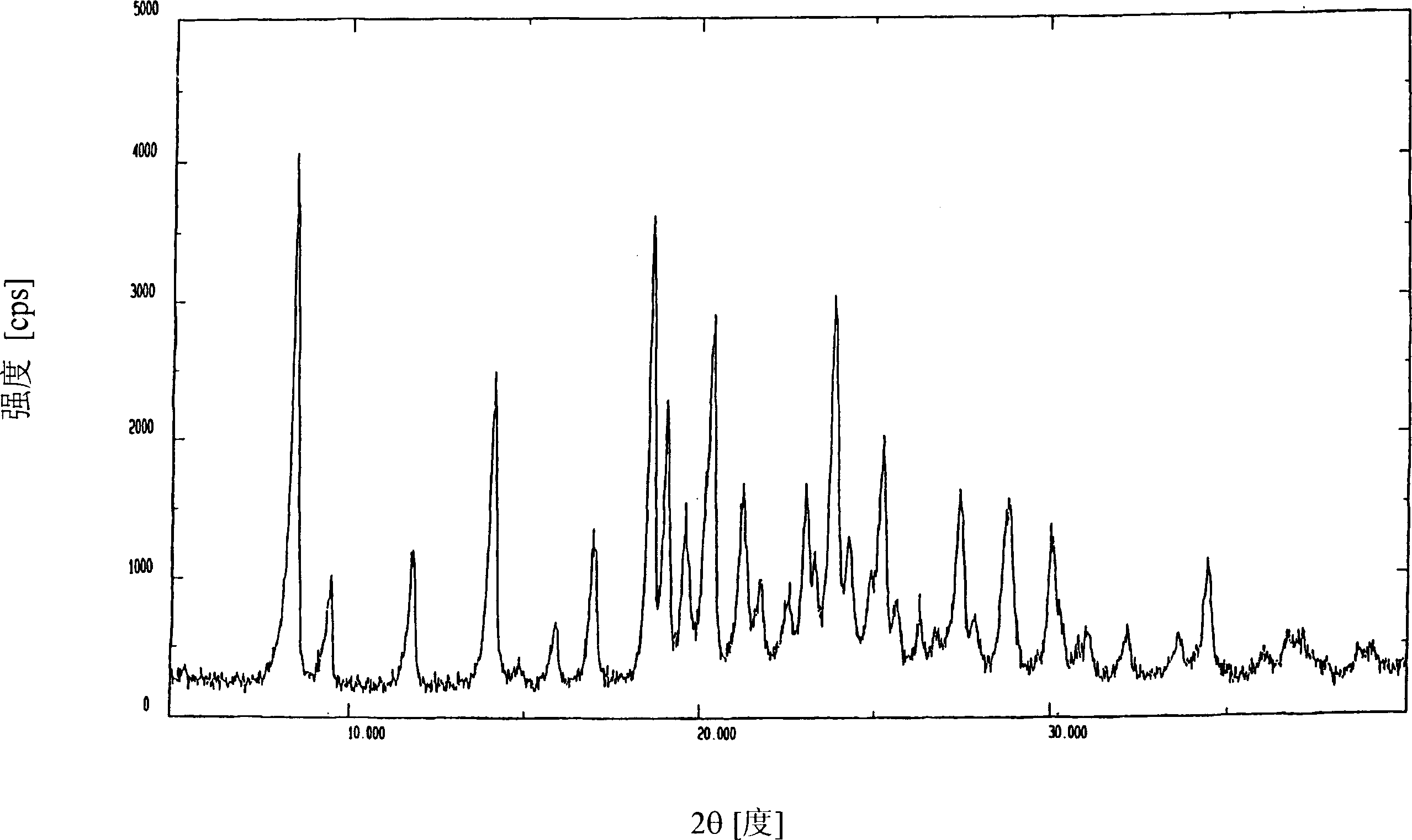
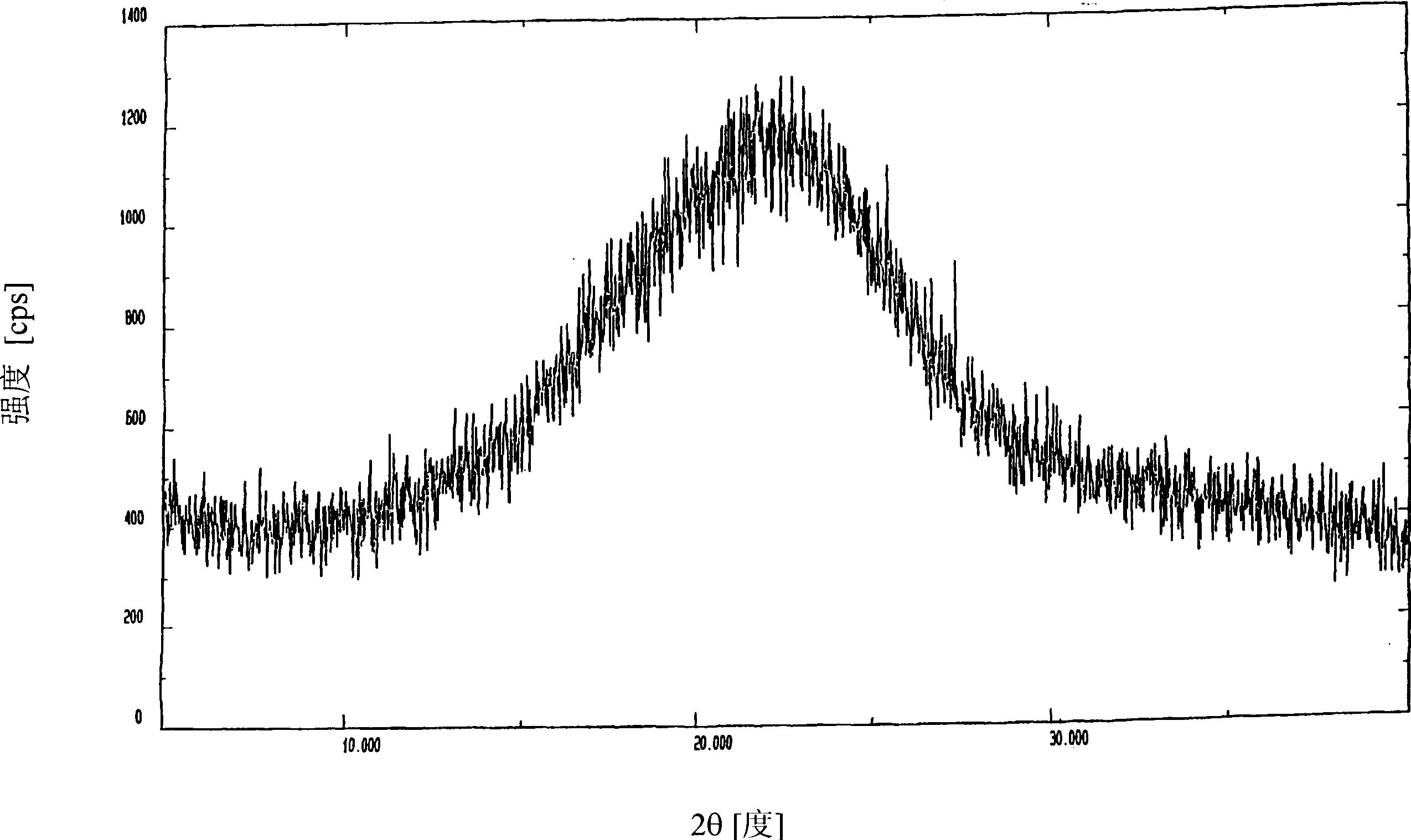
Image
Examples
reference example 1
[0035] Reference Example 1: Preparation of 7β-[(Z)-2-(2-aminothiazol-4-yl)-2-trityloxyiminoacetamido]-3-vinyl-3-cephalosporin (Cephem) -4-Carboxylic acid·p-TsOH·2DMAC
[0036] 8.0 grams of 7-amino-3-vinyl-3-cephalosporin-4-carboxylic acid and 21.5 grams of (Z)-(2-aminothiazol-4-yl)-2-trityloxyiminoacetic acid 2- Benzothiazole thioate was suspended in 80 ml of N,N-dimethylacetamide, and 16.8 ml of tri-n-butylamine was added thereto. Then, the mixture was stirred for 1 hour while maintaining a temperature of 15-20°C. 240 ml of diethyl ether was added thereto and stirred for 30 minutes, then filtered through cellite. To the filtrate was added 20.2 g of p-toluenesulfonic acid monohydrate dissolved in 40 ml of methanol, and the resulting solution was stirred at room temperature for 2 hours. After adding 160 ml of diethyl ether thereto, the resulting mixture was further stirred at room temperature for 1 hour, cooled to 0-5°C, stirred for 1 hour and filtered. The precipitate thus...
Embodiment 1
[0039] Example 1: Preparation of 7β-[(Z)-2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-vinyl-3-cephalosporin-4-carboxylic acid·H 2 SO 4
[0040]40 g of 7β-[(Z)-2-(2-aminothiazol-4-yl)-2-trityloxyiminoacetamido]-3-vinyl-3 obtained in Reference Example 1 - Cephalo-4-carboxylic acid · p-TsOH · 2DMAC was suspended in 200 mL of acetonitrile. 20 milliliters of 90% formic acid and 6.0 milliliters of 98% sulfuric acid were added thereto, and the reaction was maintained at a temperature of 15-20° C. for 20 hours. The precipitate thus obtained was filtered and washed sequentially with 100 ml of acetonitrile and 100 ml of diethyl ether, and then dried to obtain 18.2 g (yield: 91%) of the title compound as a pale yellow crystalline solid.
[0041] HPLC purity: 99.9%
[0042] E isomer: 0.08%
[0043] Melting point: 180°C (decomposition)
[0044] IR (cm -1 , KBr): 3391, 3225, 3116, 1774, 1651, 1526, 1164, 1042, 877, 672, 589, 570
[0045] H-NMR (δ, DMSO-d 6 ): 3.62, 3.85 (2H, ...
Embodiment 2
[0050] Example 2: Preparation of 7β-[(Z)-2-(2-aminothiazol-4-yl)-2-hydroxyiminoacetamido]-3-vinyl-3-cephalosporin-4-carboxylic acid·CH 3 SO 3 h
[0051] 100 g of 7β-[(Z)-2-(2-aminothiazol-4-yl)-2-trityloxyiminoacetamido]-3-vinyl-3 obtained in Reference Example 1 - Cephalo-4-carboxylic acid · p-TsOH · 2DMAC was suspended in 200 mL of acetonitrile. Add 40 milliliters of 85% formic acid and 18.5 milliliters of methanesulfonic acid therein, and then maintain the temperature of 20-25 DEG C for 20 hours. The precipitate thus obtained was filtered and washed sequentially with 100 ml of acetonitrile and 100 ml of diethyl ether, and then dried to obtain 43.9 g (yield: 88%) of the title compound as a pale yellow crystalline solid.
[0052] HPLC purity: 99.8%
[0053] E isomer: 0.12%
[0054] Melting point: 210°C (decomposition)
[0055] IR (cm -1 , KBr): 3285, 3231, 1775, 1684, 1636, 1527, 1356, 1195, 1145, 1043, 782, 590
[0056] H-NMR (δ, DMSO-d 6 ): 2.37 (3H, s, CH 3 S), 3....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com