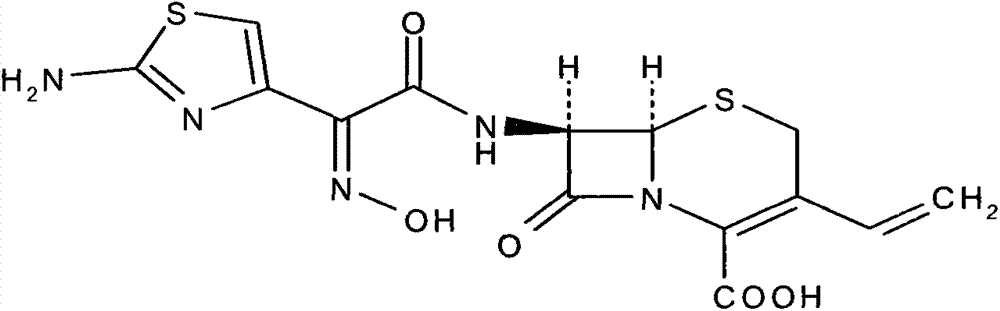
Cefdinir dispersible tablet and preparation method thereof
A technology of cefdinir and dispersible tablets, which is applied in the field of cefdinir dispersible tablets and its preparation, can solve the problems of large dosage, limitations, and low drug loading, and achieve good dispersion uniformity and quality stability, The effect of improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
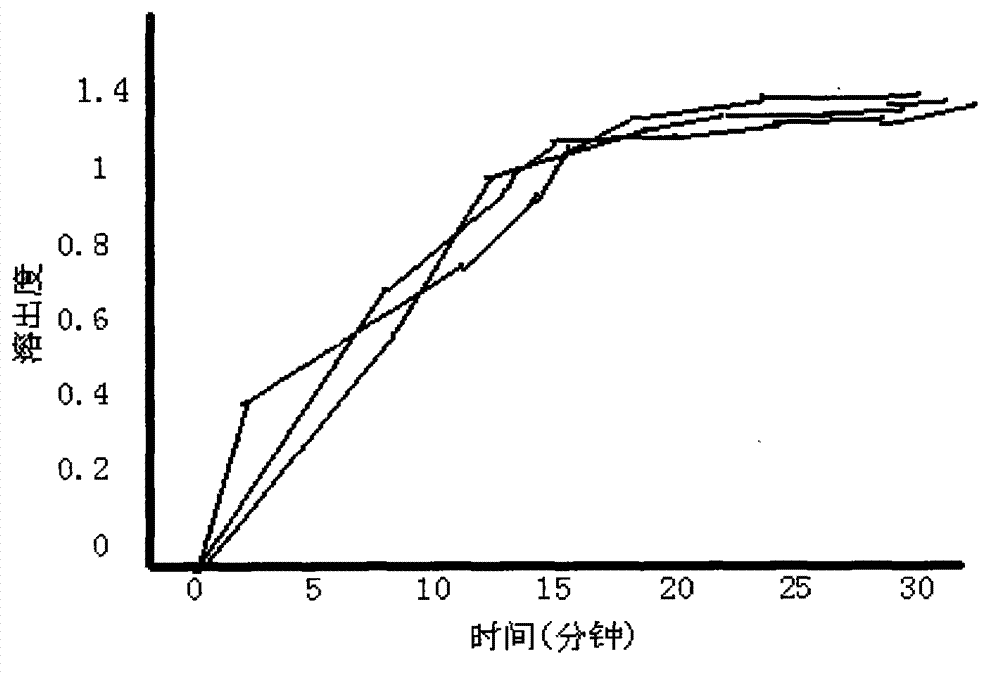
Embodiment 1
[0032]
Embodiment 2
[0034]
Embodiment 3
[0036]
[0037] The preparation method of embodiment one~three
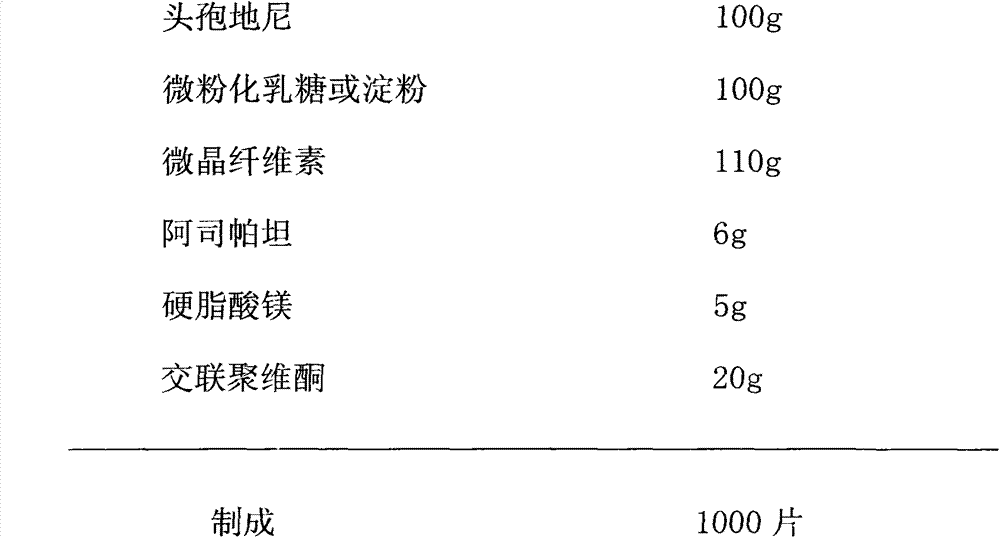
[0038] (1) Micronize the cefdinir raw material into powder with a particle size of 60-150 μm, and pass the rest of the auxiliary materials through a 100-mesh sieve for subsequent use;
[0039] (2) Mix cefdinir, micronized lactose or starch, microcrystalline cellulose, aspartame and 30% magnesium stearate in prescription quantity;
[0040] (3) Extrude into block with dry method, crush, granulate with 18 mesh sieves, granulate with 16 mesh sieves; add magnesium stearate and crospovidone of recipe quantity 70%, mix homogeneously;
[0041] (4) Determination of intermediate content;
[0042] (5) Compressed into tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com