Compound cefaclor suspension and preparation method thereof
The technology of cefaclor and suspension, which is applied in the field of pharmaceutical preparations, can solve the problems of poor solubility, slow onset of drug effect and the like, and achieve the effects of stable drug properties, fast onset of effect and easy portability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
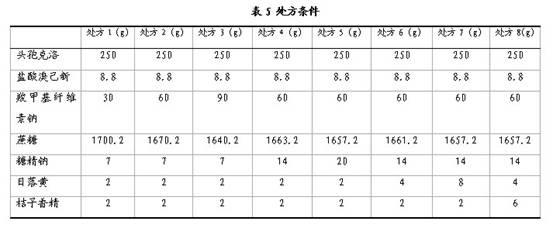
[0045] Compound cefaclor dry suspension, calculated in 1000 bags, its prescription composition:
[0046]
[0047] Taking the preparation of 1000 bags as an example, the preparation method of the above-mentioned compound cefaclor dry suspension is as follows:
[0048] Weigh the prescription amount of sodium saccharin and sunset yellow, add water and stir to dissolve, then add an appropriate amount of 95% ethanol, stir evenly for later use; pass sodium carboxymethyl cellulose and sucrose through an 80-mesh sieve for later use; mix sodium carboxymethyl cellulose and sucrose After mixing, add the above-mentioned ethanol-water solution containing sodium saccharin and sunset yellow to make a soft material, pass through a 30-mesh sieve to granulate, dry at 60°C, and pass the dry granules through a 30-mesh granule; mix the granules with cefaclor and bromine hydrochloride The same amount of Jixin is added and mixed, and finally orange essence is added, mixed evenly, and then packed ...
Embodiment 2
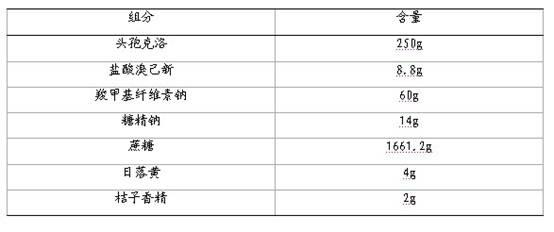
[0056] Compound cefaclor dry suspension, calculated in 1000 bags, its prescription composition:
[0057]
[0058] Weigh the prescription amount of sodium saccharin and sunset yellow, add water and stir to dissolve, then add an appropriate amount of 95% ethanol, stir evenly for later use; pass sodium carboxymethyl cellulose and sucrose through an 80-mesh sieve for later use; mix sodium carboxymethyl cellulose and sucrose After mixing, add the above-mentioned ethanol-water solution containing sodium saccharin and sunset yellow to make a soft material, pass through a 30-mesh sieve to granulate, dry at 60°C, and pass the dry granules through a 30-mesh granule; mix the granules with cefaclor and bromine hydrochloride Add the same amount of Jixin and mix it evenly, and finally add orange essence and micro-powdered silica gel, mix it evenly, and pack it separately (1g / bag).
Embodiment 3
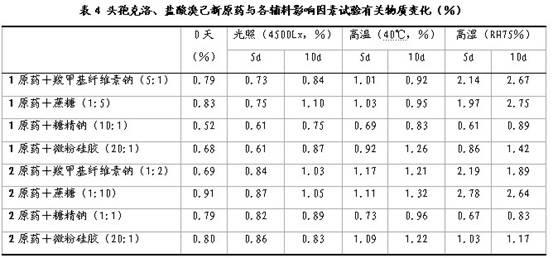
[0060] Mix cefaclor (1 original drug), bromhexine hydrochloride (2 original drug) and related excipients in a certain proportion and then granulate them. The prepared granules are subjected to strong light (4500Lx), high temperature (40°C) Humidity (RH75%) influencing factors investigation, the results are shown in Table 4. It can be seen from Table 4 that the investigation results of the influencing factors of the original drug and various excipients show that its stability is good.
[0061]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com