Separation and purification method for cefaclor by enzymatic synthesis
A cefaclor and enzymatic synthesis technology, applied in organic chemistry, fermentation, etc., can solve the problems of inconvenient batch purification, large amount of solvent usage, harsh reaction conditions, etc., to reduce side chain consumption, increase batch feeding, and stable quality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
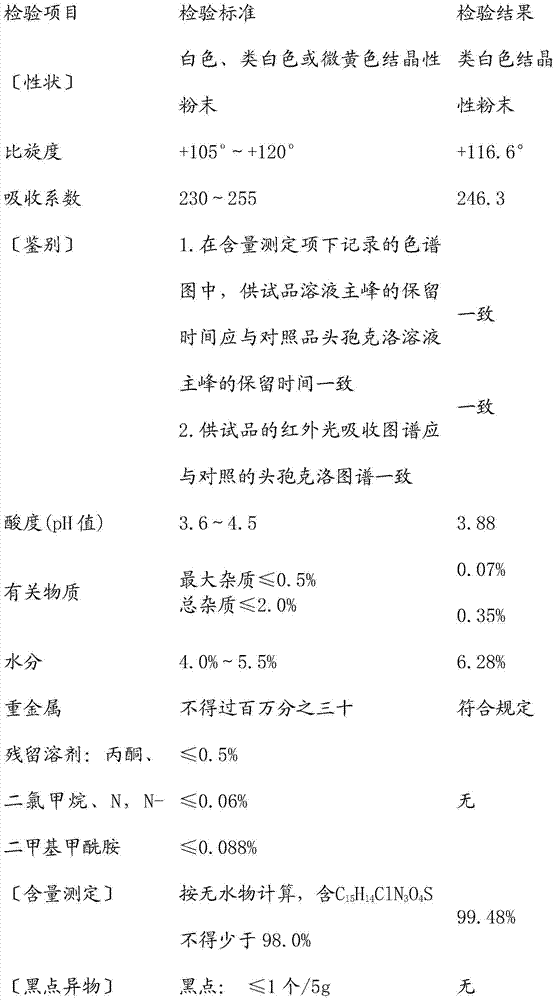
[0024] Embodiment 1: A kind of separation and purification method of enzymatic synthesis of cefaclor
[0025] In a 1000ml enzyme reactor, add 300ml of water, 25g of 7-amino-3-chlorocephalosporanic acid (7-ACCA), control the temperature at 11~20°C, add ammonia solution dropwise to pH 7.0±0.1, stir to dissolve, add fixed Add 25g of enzyme, and then drop 24g of D-phenylglycine methyl ester hydrochloride solution through the dropping funnel to obtain the enzymatic reaction system, and then use the pH automatic control device to control the pH value of the enzymatic reaction system to be constant at 6.5~6.8. The immobilized enzyme was separated once in 30 minutes and reacted to generate cefaclor. The separation method is to pass the enzymatic reaction solution through a 125-micron sieve, the oversize, that is, the immobilized enzyme, is returned to the enzyme-catalyzed reaction system, and the undersize is filtered to obtain the filtrate and filtrate, and the filtrate is washed and...
Embodiment 2
[0029] Embodiment 2: A kind of separation and purification method of enzymatic synthesis of cefaclor
[0030] In a 1000ml enzyme reactor, add 500ml H 2 O, 30g of 7-ACCA, control the temperature at 18~22℃, add ammonia water dropwise to pH 7.0±0.1, stir to dissolve, add 20g of immobilized enzyme, add 37g of D-phenylglycine methyl ester methanesulfonate solution dropwise through the dropping funnel to obtain Enzyme-catalyzed reaction system, the pH value of the reaction is controlled by the pH automatic control device to be constant at 6.5~6.8, cefaclor is continuously precipitated as the reaction progresses, continuous separation and circulation, the precipitated cefaclor is collected, and the mother liquor is recycled back to the enzyme reactor, 3.5 hours or so. Specifically, the pipeline is connected from the 1000ml enzyme reactor. The pipeline first leads to a screen, a filter device is arranged under the screen, a filtrate collection bucket is arranged under the filter devi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com