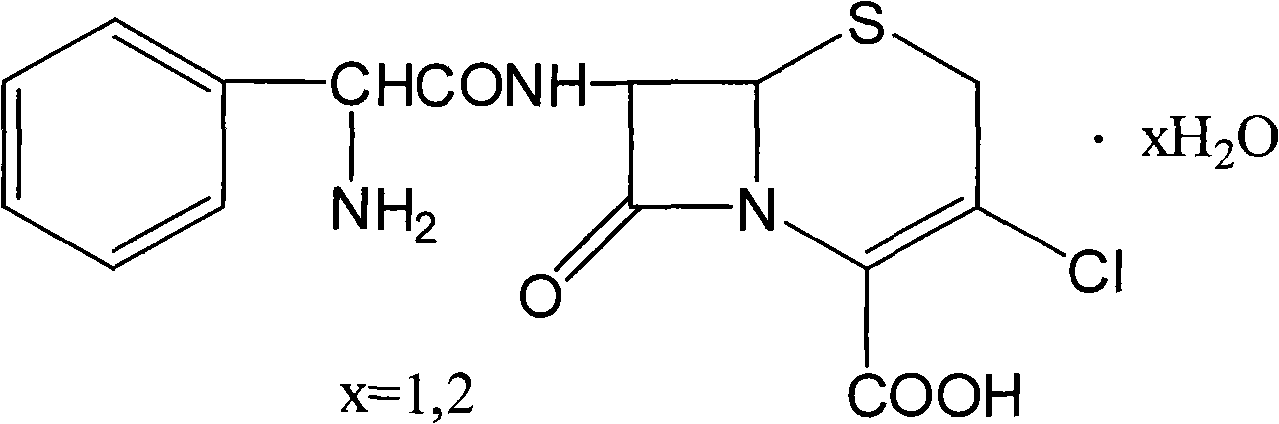
Method for extracting cefaclor from cefaclor naphthalenol complexes
A technology of faclor naphthol and cefaclor, applied in the field of purifying cefaclor, can solve problems such as affecting the yield of cefaclor, and achieve the effect of low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 , preparation of cefaclor naphthol compound
[0025] With reference to the method of WO2006 / 069984, the cefaclor naphthol complex was prepared, specifically as follows:
[0026] Weigh 19.15 g of 7-amino-3-chloro-desacetoxy-cephalosporanic acid (7-ACCA), add about 500 ml of deionized water, add ammonia water dropwise to completely dissolve 7-ACCA, and slowly adjust the pH to 6.4 with sulfuric acid.
[0027] Weigh 33.62g of D-phenylglycine methyl ester (PGME), add a small amount of water to dissolve completely, add PGME to the 7-ACCA solution, mix well, add phosphate buffer to adjust the pH to 6.4, add deionized water to 1000ml .
[0028] Connect the pipelines of the in-situ separation reactor, and control the temperature of the reactor to 20°C. Then add about 75% of the above substrate solution into the reaction bottle, and about 25% of the substrate solution into the complexation bottle. Then, 20.8 g (about 4000 IU) of immobilized penicillin acylase (IPA75...
Embodiment 2
[0029] Example 2 , Purify Cefaclor from Cefaclor Naphthol Complex
[0030] 2.1. Dissolution and preliminary purification of cefaclor naphthol complex
[0031] With a total amount of 400-450 ml of water, wash the cefaclor naphthol complex several times, collect the washing liquid, adjust the pH value to about 1.5 with sulfuric acid or hydrochloric acid, then add an equal volume of butyl acetate, and stir for 1 hour . Stand still for 15-30 minutes, after the layers are separated, the water phase is collected and the butyl acetate phase is preserved. Add an equal volume of butyl acetate to the water phase again, adjust the pH value to about 1.5, stir for 1 hour, and after standing to separate layers, collect the water phase and save the butyl acetate phase. Combine the butyl acetate phases and add 100-150 ml of water to adjust the pH to about 1.5. Stir for 1 hour, after standing for stratification, collect the water phase, combine the water phases, and detect the content of ...
Embodiment 3
[0043] Example 3 ,crystallization
[0044] The cefaclor eluate purified by the macroporous adsorption resin was evaporated under reduced pressure at 35-45°C, concentrated, then added an equal volume of isopropanol to stir, cooled at 4°C, and slowly added ammonia water to the solution Turbidity began to appear. After stirring for 1 hour, adjust the pH value to 4 with ammonia water, stir evenly, and obtain cefaclor hydrate solid after crystallization and drying. At the same time, crystallize with the mixed aqueous solution of cefaclor and impurities obtained in step 2.1 as a control .
[0045] The purity of cefaclor and the content of each main impurity in the solid obtained from the cefaclor hydrate crystal obtained above were detected respectively, and the results are shown in Table 3.
[0046] Table 3, the purity of cefaclor in crystals and the detection results of main impurities
[0047]
#
Cefaclor
Yield
Cefaclor
purity
Pheny...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com