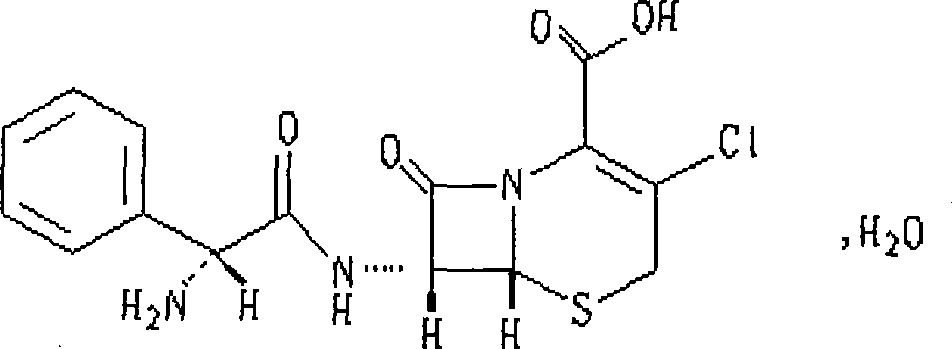
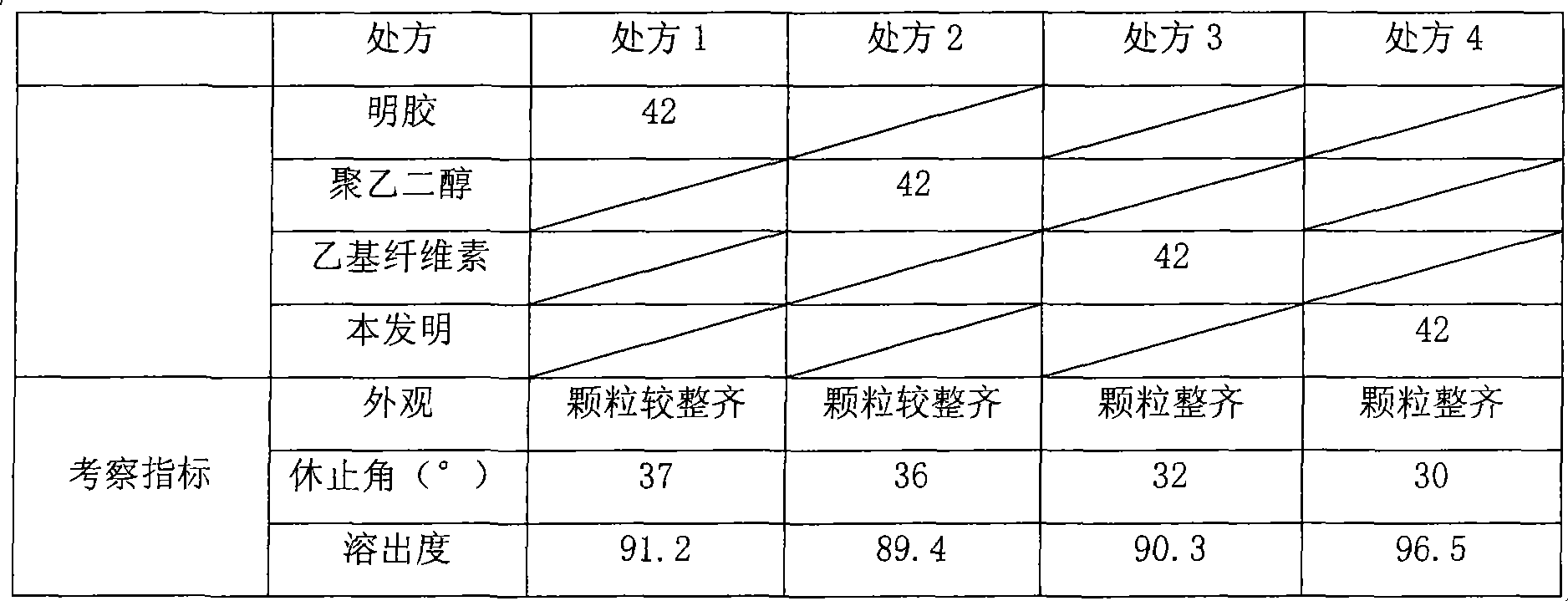
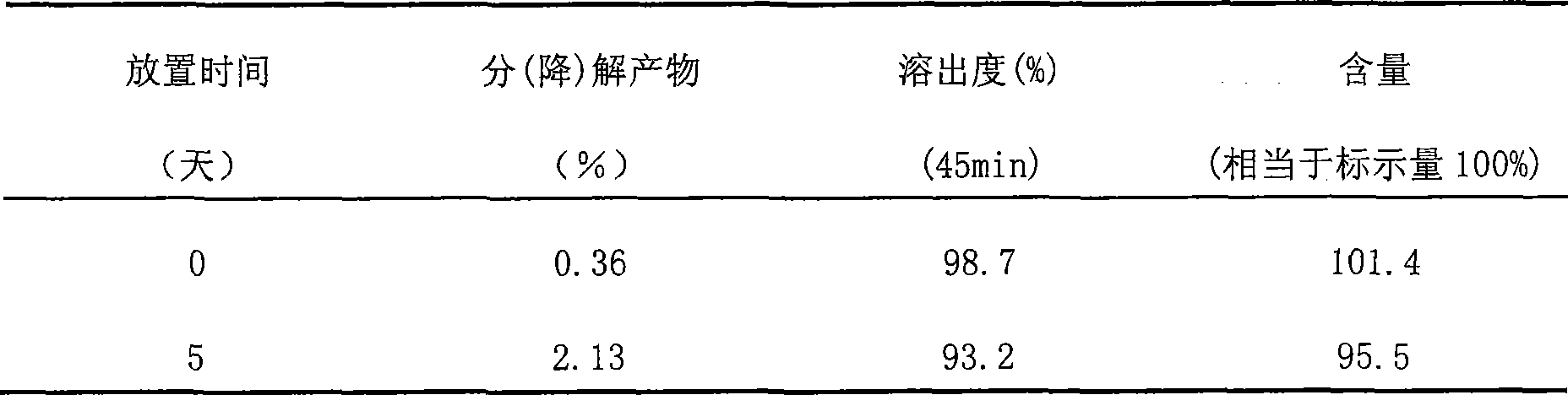
Cefaclor orally disintegrating tablet and preparation method thereof
A technology of orally disintegrating tablets and cefaclor, which is applied in the field of medicine, can solve the problems of reducing patient treatment compliance, and achieve the effects of increasing bioavailability, increasing stability, and improving bioavailability and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] The content of each component per thousand tablets is as follows:
[0058] Cefaclor…………………………………………………………… 250g
[0059] Polyacrylic resin IV……………………………………………… 30g
[0060] NE30D…………………………………………………… 3g
[0061] Ethyl cellulose…………………………………………………………………………………………30g
[0062] Mannitol………………………………………………………………… 120g
[0063] Cross-linked polyvinylpyrrolidone………………………………………………… 20g
[0064] Low-substituted hydroxypropyl cellulose…………………………………………… 10g
[0065] Micropowder silica gel…………………………………………………………………………3g
[0066] Aspartame………………………………………………………… 10g
[0067] Magnesium stearate………………………………………………………………………………………………5g 5g
[0068] Preparation:
[0069] ①. The polyacrylic resin IV, Dissolve NE30D and ethyl cellulose in an appropriate amount of ethanol and stir thoroughly to dissolve, then add 20% of the prescription amount of micropowder silica gel, keep stirring to suspend the micropowder silica gel evenly, and use it as a capsule material for later use;
[0070]...
Embodiment 2
[0076] The content of each component per thousand tablets is as follows:
[0077] Cefaclor………………………………………………………………… 125g
[0078] Polyacrylic resin IV…………………………………………… 5g
[0079] NE30D………………………………………………… 1g
[0080] Ethyl cellulose………………………………………………………………………5g
[0081] Mannitol……………………………………………………………40g
[0082] Cross-linked polyvinylpyrrolidone……………………………………… 5g
[0083] Low-substituted hydroxypropyl cellulose…………………………………………… 2g
[0084] Micropowder silica gel………………………………………………………………………………………………………………………………………1g
[0085] Aspartame ……………………………………………………… 2g
[0086] Magnesium stearate…………………………………………………………………………… 2g
[0087] Preparation:
[0088] ①. The polyacrylic resin IV, Dissolve NE30D and ethyl cellulose in an appropriate amount of ethanol and stir thoroughly to dissolve, then add 20% of the prescription amount of micropowder silica gel, keep stirring to suspend the micropowder silica gel evenly, and use it as a capsule material for later use;
[0089] ②. Mi...
Embodiment 3
[0095] The content of each component per thousand tablets is as follows:
[0096] Cefaclor………………………………………………………… 62.5g
[0097] Polyacrylic resin IV…………………………………………… 5g
[0098] NE30D…………………………………………………… 0.5g
[0099] Ethyl cellulose………………………………………………………………………5g
[0100] Mannitol……………………………………………………………… 25g
[0101] Cross-linked polyvinylpyrrolidone……………………………………… 4g
[0102] Low-substituted hydroxypropyl cellulose…………………………………………… 2g
[0103] Micropowder silica gel…………………………………………………………………………………………………………………………………………………………………………………………………………………0.6g
[0104] Aspartame ……………………………………………………… 2g
[0105] Magnesium stearate ……………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………………1g
[0106] Preparation:
[0107] ①. The polyacrylic resin IV, Dissolve NE30D and ethyl cellulose in an appropriate amount of ethanol and stir thoroughly to dissolve, then add 20% of the prescription amount of micropowder silica gel, keep stirring to suspend...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com