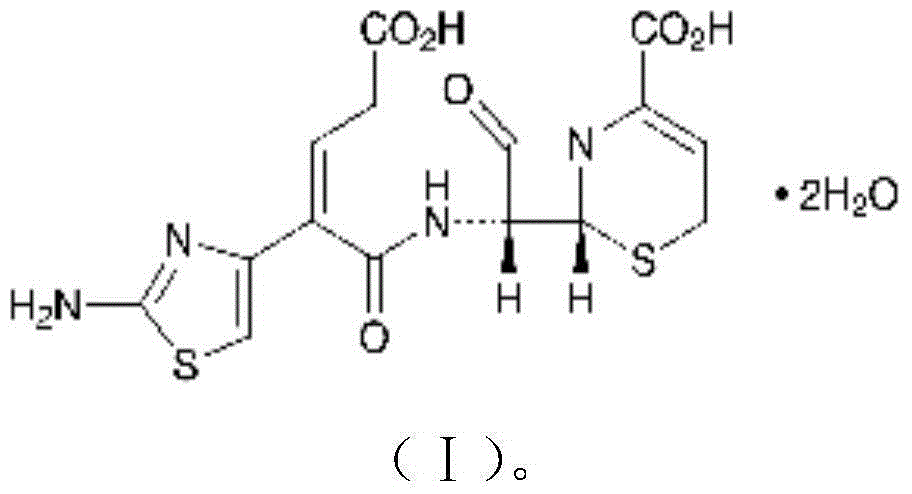
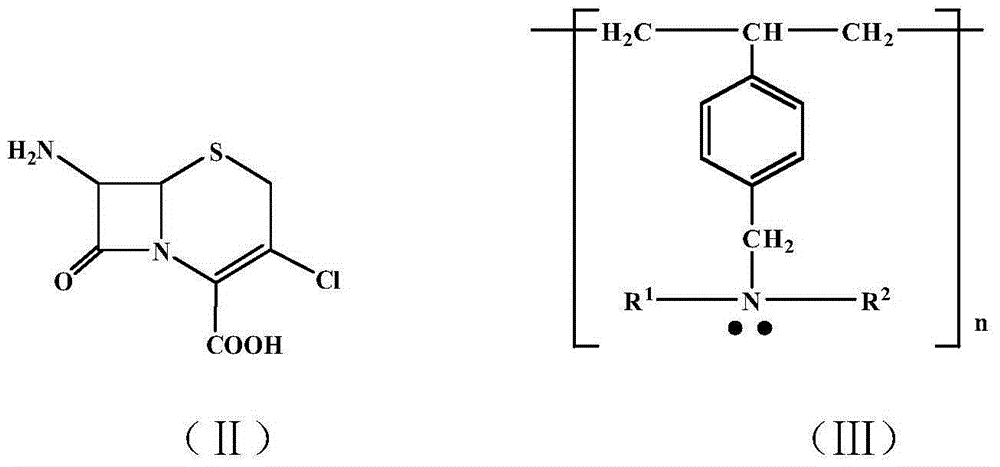
Preparation method of ceftibuten
A technology of cefbutene and cefaclor mother nucleus, which is applied in the field of medicine and chemical industry and achieves the effects of high yield, simple operation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] In a 100 ml three-necked flask, add 2.4 grams (0.01 moles) of cefaclor core, 15 milliliters of methyl tetrahydrofuran, and 0.4 grams (0.017 moles) of magnesium powder. After reacting at 30°C until the magnesium powder disappears, add 10 milliliters of distilled water, Stir for 10 minutes, stand to separate the layers, separate the methyl tetrahydrofuran organic layer and dry it with anhydrous magnesium sulfate. After filtering, add 0.7 g of Styrene-DVB (D301R) resin, 2-(2-benzyloxycarbonylaminothiazole-4- base)-5-benzyloxycarbonyl-2-pentenoic acid 5 g (0.011 moles), reacted at 30°C for 3 hours, filtered off the Styrene-DVB (D301R) resin after the reaction, distilled off methyl tetrahydrofuran, and added iso 10 ml of isopropanol and 20 ml of NaOH (30%) aqueous solution were reacted at 30°C for 1 hour, then cooled to 20°C. Extracted three times with 10×3 ml of dichloromethane, added concentrated HCl dropwise to the aqueous solution layer at 0°C to adjust the pH=5-6, left ...
Embodiment 2
[0027] In a 100 ml three-necked flask, add 2.4 grams (0.01 moles) of cefaclor core, 15 milliliters of methyl tetrahydrofuran, and 0.4 grams (0.017 moles) of magnesium powder. After reacting at 50 ° C until the magnesium powder disappears, add 10 milliliters of distilled water, Stir for 10 minutes, stand to separate the layers, separate the methyl tetrahydrofuran organic layer and dry it with anhydrous magnesium sulfate. After filtering, add 1.2 grams of Styrene-DVB (D301R) resin, 2-(2-benzyloxycarbonylaminothiazole-4- base)-5-benzyloxycarbonyl-2-pentenoic acid 5 g (0.011 moles), reacted at 30°C for 1 hour, filtered off the Styrene-DVB (D301R) resin after the reaction, distilled off methyl tetrahydrofuran, and added iso 10 ml of isopropanol and 20 ml of NaOH (30%) aqueous solution were reacted at 30°C for 1 hour, then cooled to 20°C. Extracted three times with 10×3 ml of dichloromethane, added concentrated HCl dropwise to the aqueous solution layer at 0°C to adjust the pH=5-6, ...
Embodiment 3
[0029] In a 100 ml three-necked flask, add 2.4 grams (0.01 moles) of cefaclor core, 15 milliliters of methyl tetrahydrofuran, and 0.4 grams (0.017 moles) of magnesium powder. After reacting at 30°C until the magnesium powder disappears, add 10 milliliters of distilled water, Stir for 10 minutes, stand to separate the layers, separate the methyl tetrahydrofuran organic layer and dry it with anhydrous magnesium sulfate. After filtering, add 0.7 g of Styrene-DVB (D301T) resin, 2-(2-benzyloxycarbonylaminothiazole-4- base)-5-benzyloxycarbonyl-2-pentenoic acid 5 g (0.011 moles), reacted at 10°C for 3 hours, filtered off Styrene-DVB (D301T) resin at the end of the reaction, distilled off methyl tetrahydrofuran, and added iso 10 ml of isopropanol, 20 ml of NaOH (30%) aqueous solution, reacted at 20°C for 1 hour, extracted three times with 10×3 ml of dichloromethane, added concentrated HCl dropwise at 0°C to the aqueous solution to adjust the pH to 5-6, and statically Crystals were pre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com