Method for preparing cefaclor dispersible tablets by dry method direct tablet compressing and cefaclor dispersible tablets prepared by same
A technology of cefaclor and dispersible tablets, which is applied in the direction of medical formulations, organic active ingredients, and medical preparations of non-effective ingredients, etc., and can solve the problems of cefaclor dispersible tablets stability, many process steps, and large drug loss. , to achieve the effect of short cycle, simple equipment and small drug loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of the present invention is to prepare cefaclor dispersible tablets by direct compression of dry powder. The so-called direct compression of dry powder is to mix the raw drug powder with the processed adjuvant without going through steps such as making soft materials, granulating, and drying. Directly carry out tabletting; In the present invention, at first cefaclor crude drug and auxiliary material are passed through 80 mesh sieves respectively, can screen out larger particle and crystal like this, the fineness after bulk drug and auxiliary material crossed 80 mesh sieve just has It is beneficial to mix uniformly; then each auxiliary material is dried separately, then cooled to room temperature, then each auxiliary material is mixed with the cefaclor bulk drug in a three-dimensional mixer, and finally the mixed material can be directly compressed into tablets. The preparation of cefaclor of the present invention The method of Luo dispersible table...
Embodiment 1
[0030] Weigh 125g of raw material cefaclor, 290g of microcrystalline cellulose, 30g of sodium starch glycolate, 15g of micropowder silica gel, 20g of talcum powder, and 5g of magnesium stearate as auxiliary materials, and pass through 80-mesh sieve respectively; Dry and pretreat in an oven for 4 hours, control the moisture content below 2% and cool to room temperature; fully mix the pretreated excipients and cefaclor raw material in a three-dimensional mixer for 40 minutes, and then directly compress into tablets, and prepare 1000 tablets in total .
Embodiment 2
[0032] Take by weighing 250g of raw material cefaclor, 210g of microcrystalline cellulose, 26g of sodium starch glycolate, 15g of micropowder silica gel, 15g of talcum powder, and 2.5g of magnesium stearate as auxiliary materials; respectively pass through a 80-mesh sieve. Dry and pretreat the excipients in an oven at 50-70°C for 5 hours, keep the moisture below 2% and cool to room temperature; fully mix the pretreated excipients and cefaclor raw material in a three-dimensional mixer for 40 minutes, and directly Compression, a total of 1000 tablets were prepared.
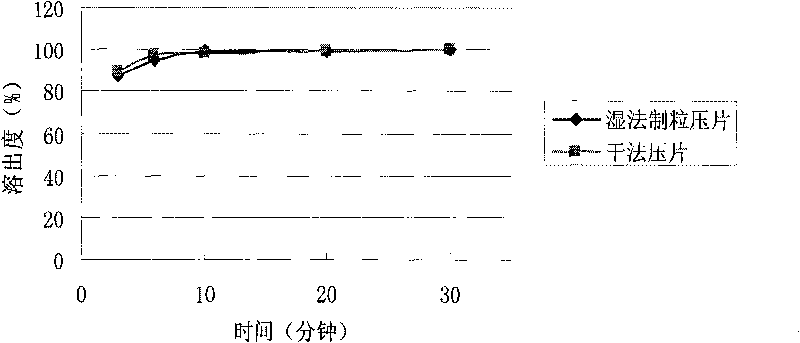
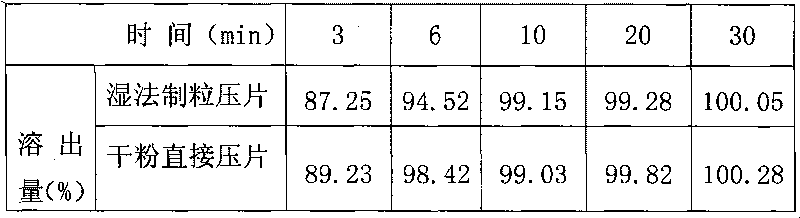
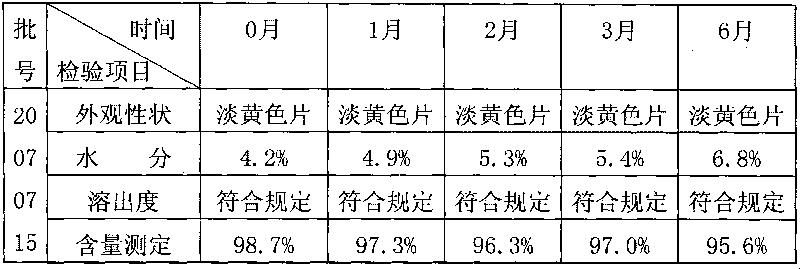
[0033] The following is the comparison of the cefaclor dispersible tablet prepared by the dry direct compression method of the present invention and the cefaclor dispersible tablet prepared by the traditional wet granulation tabletting method.
[0034] Table 1: Effect of preparation process on cefaclor dispersible tablets
[0035] Preparation
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com