Sustained-release composition of cefaclor
A composition and technology of cephalosporins, which are applied in drug delivery, medical preparations of non-active ingredients, organic active ingredients, etc., can solve the problems of long complete release time and affecting curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
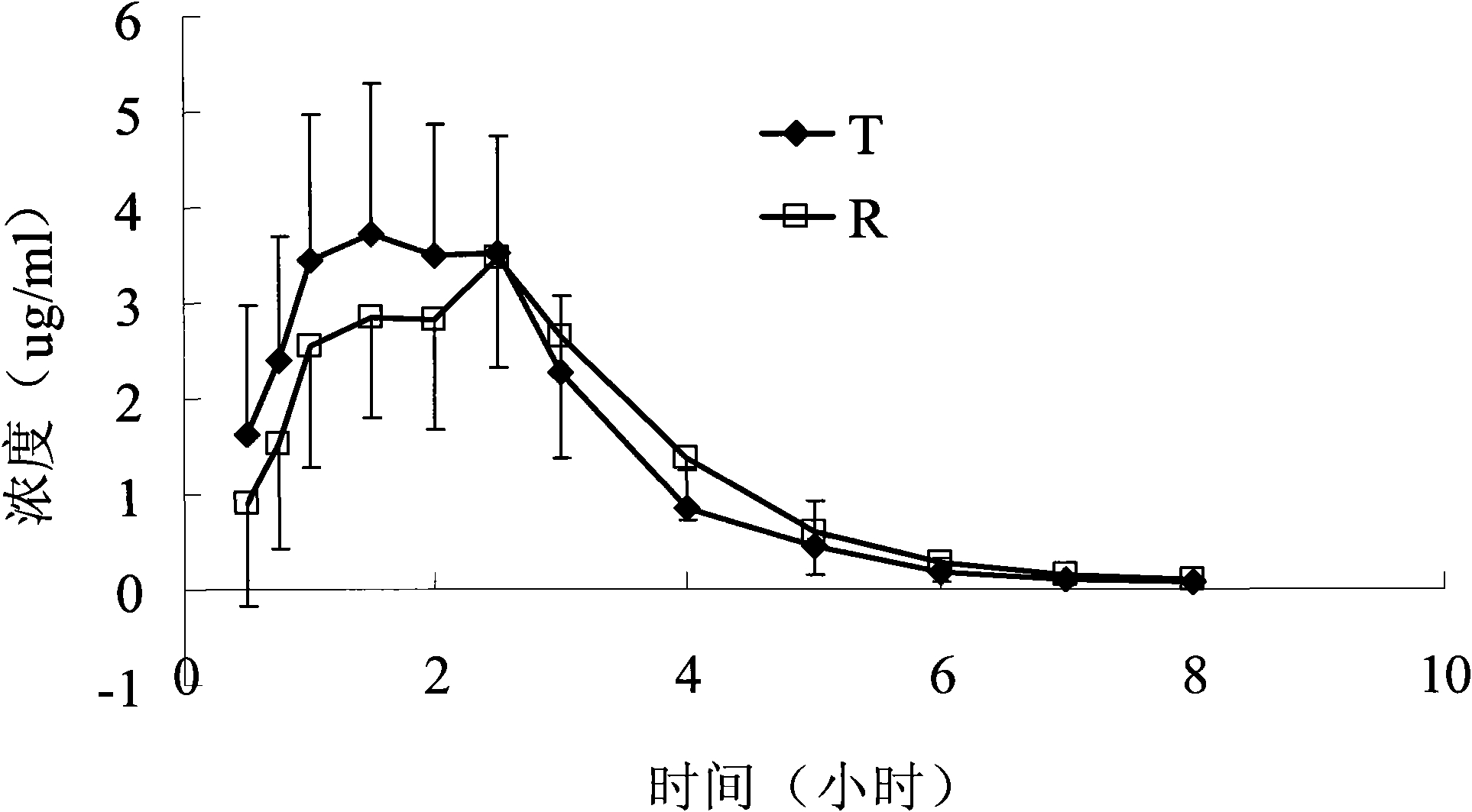
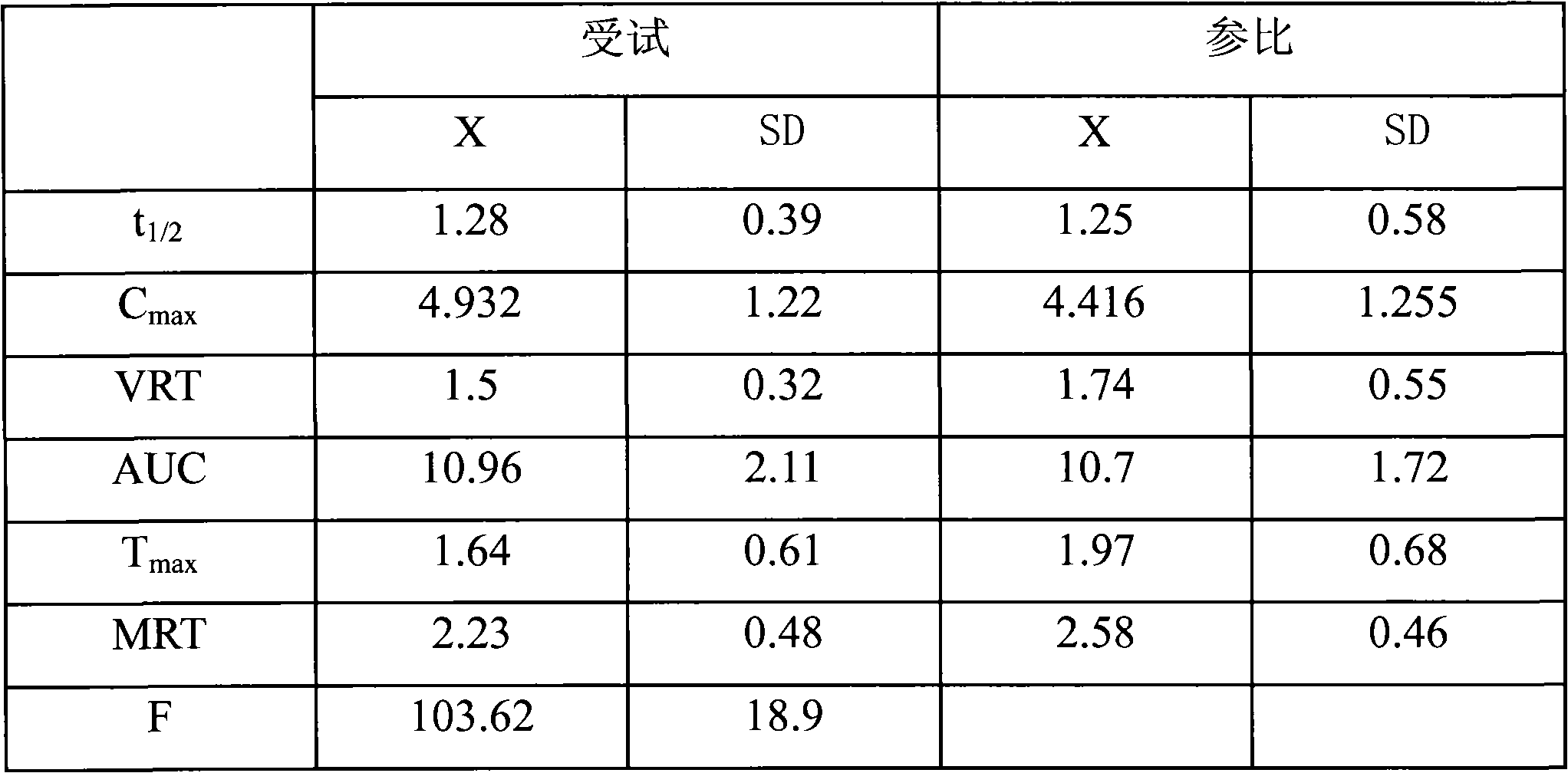
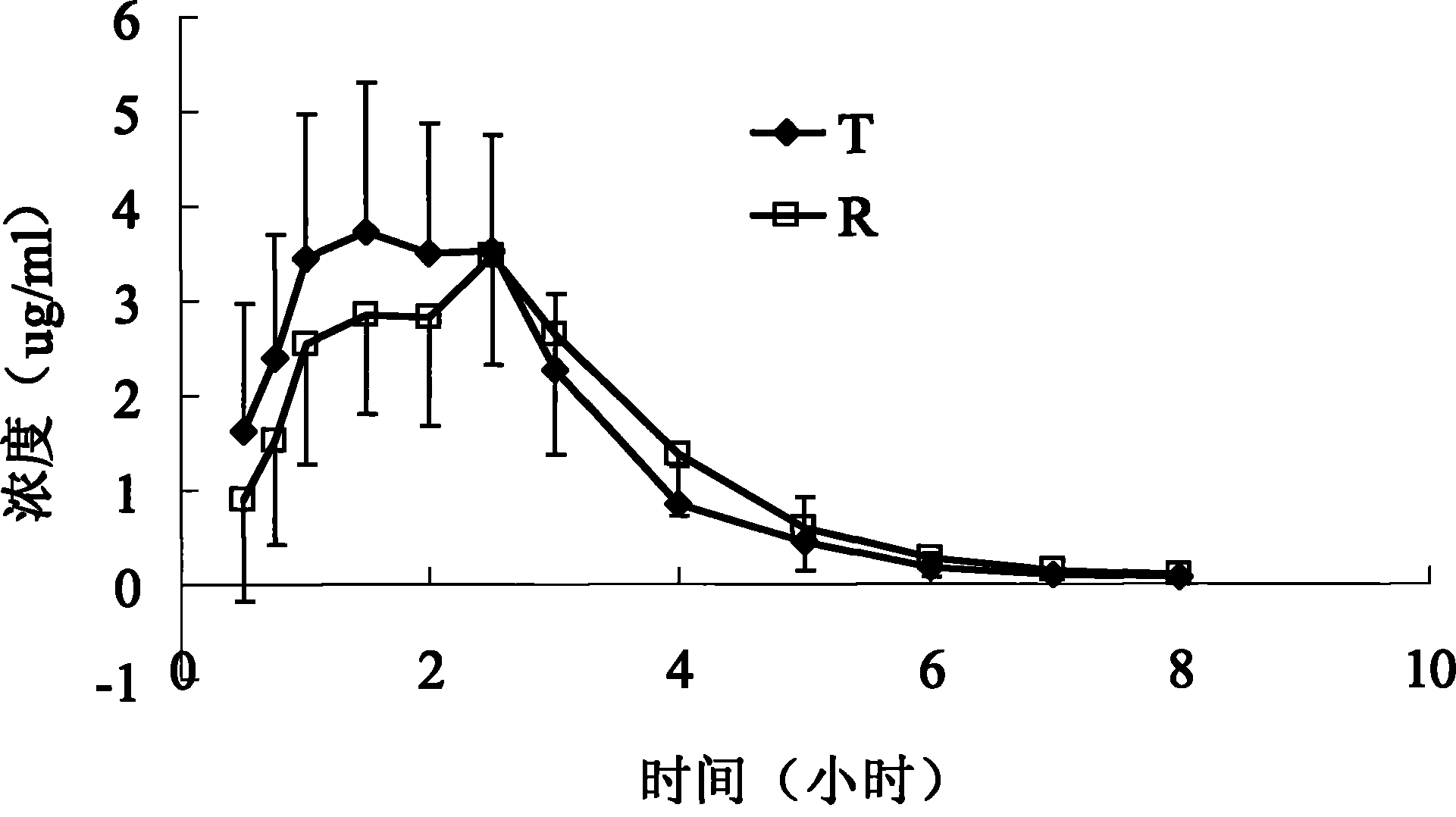
Embodiment 1
[0020] Raw materials (g)
[0021] Take cefaclor, sodium carboxymethyl cellulose, mannitol, povidone (K30), hypromellose (E5), and microcrystalline cellulose, sieve and mix well, and use 5% polyacrylic acid resin II ethanol solution Soft material, 16-mesh granulation, drying, 12-mesh granulation, adding magnesium stearate, mixing, tableting.
Embodiment 2
[0023] Raw materials
[0024] Sodium carboxymethyl cellulose
[0025] Take cefaclor, sodium carboxymethylcellulose, mannitol, hypromellose (K100M), and stearic acid, sieve and mix well, use 5% polyacrylic resin II ethanol solution to make soft material, and granulate with 16 mesh , dried, 12-mesh granules, added magnesium stearate, mixed evenly, and compressed into tablets.
Embodiment 3
[0027] Raw materials
[0028] Take cefaclor, sodium carboxymethylcellulose, sucrose, hypromellose (E5), hypromellose (100M) and stearic acid, sieve and mix thoroughly, and use 5% polyacrylic acid resin II ethanol solution Soft material, 16-mesh granulation, drying, 12-mesh granulation, adding magnesium stearate, mixing, tableting.
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com