Induced Hepatic Stem Cell And Process For Production Thereof, And Applications Of The Cell
A technology for hepatic stem cells and hepatocytes is applied in the fields of induced hepatic stem cells and their manufacture, as well as the uses of the cells, and can solve the problems of inability to manufacture cells and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] 1. Preparation of pantropic retroviral vector
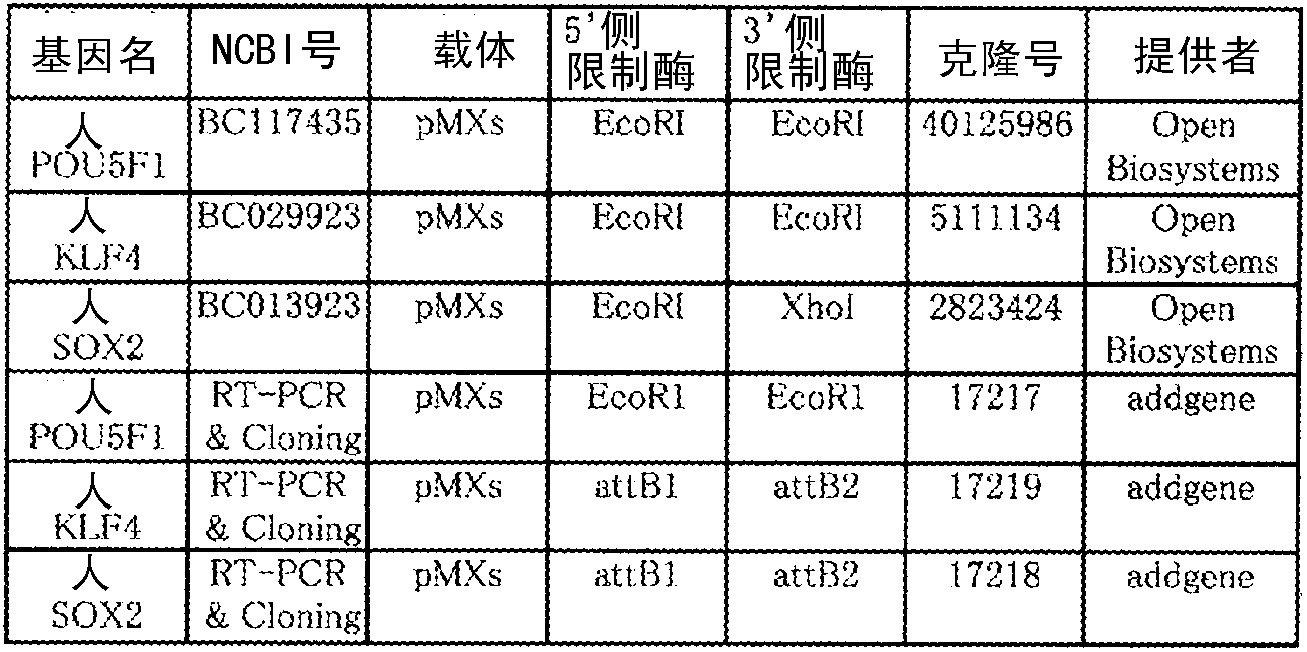
[0136] Using Fugene HD (manufactured by Roche; Cat no.4709691), the retroviral vector plasmids of the three genes POU5F1-pMXs, KLF4-pMXs, and SOX2-pMXs were introduced into packaging cells for the preparation of pantropic retroviral vectors Plat-GP cells to prepare retroviral vector solution. The genes POU5F1-pMXs, KLF4-pMXs, SOX2-pMXs were used in a ratio of 4:2:1 in sequence. The ratio of 4:2:1 can be used to introduce genes into packaging cells. In addition, it can also be used to prepare retroviral vector solutions of the three genes POU5F1-pMXs, KLF4-pMXs, and SOX2-pMXs separately and use 4: Mix at a ratio of 2:1. details as follows.
[0137]
[0138] The addgene vector was used for the genes POU5F1-pMXs, KLF4-pMXs, and SOX2-pMXs (Table 5 below).
[0139] The amount of each vector was set to POU5F1-pMXs (manufactured by addgene) 2μg, KLF4-pMXs (manufactured by addgene) 1μg, SOX2-pMXs (manufactured by addgene) 0.5μg, Ve...
Embodiment 2
[0157] 2. Produce human-induced liver stem cells from cells derived from neonatal skin tissue
[0158] Human-induced hepatic stem cells are produced from cells derived from human newborn skin tissues (trade name: neonatal normal human skin fibroblasts; primary culture; Lot no. 7F 3956) belonging to tissues just after birth.
[0159] One freeze-preserved cell derived from human newborn skin tissue (primary culture; manufactured by Lonza; CC-2511; Lot no.7F3956) was thawed in a constant temperature bath at 37°C and suspended in a thermostat containing 1X antibiotic-antifungal (Invitrogen; Cat no. 15240-062) and 10% FBS D-MEM (high glucose) (Invitrogen; Cat no. 11965-092) medium to obtain 10 ml of cell suspension.
[0160] Next, the obtained cell suspension was centrifuged at 1000 rpm and 4°C for 5 minutes. After removing the supernatant, it was suspended in Fibroblast Medium Kit-2 (2% FBS) (hereinafter also referred to as registered trademark FGM) -2BulletKit) (manufactured by Lonza...
Embodiment 3
[0192] 3. Produce human-induced liver stem cells from cells derived from cancer tissues of patients with gastric cancer
[0193] Cells are separated from cancerous tissues of human gastric cancer (advanced cancer) patients. Three genes (the ratio of POU5F1, KLF4, and SOX2 are 4:2:1) were added to the obtained cells to carry out gene transfer to produce human induced hepatic stem cells. details as follows.
[0194] Use Hank's balanced salt solution (without phenol red) (manufactured by Invitrogen; Cat no.14175-095) to wash a part of the fresh tissue of gastric cancer (advanced cancer: male, 67 years old, Japanese) obtained during surgery, thin with scissors Cut into about 1mm 2 . Furthermore, wash with Hank's balanced salt solution (without phenol red) until the supernatant becomes transparent, remove the supernatant, and add 5 ml of 0.1% collagenase (manufactured by Wako Pure Chemical Industries, Ltd.; Cat no.034-10533) / 1X antibiotic to the tissue pellet. An antifungal agent (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com