Electronic medical record big data-based clinical reasonable drug use risk assessment method
An electronic medical record and risk assessment technology, applied in data processing applications, electronic digital data processing, special data processing applications, etc., can solve the problems of low intelligence of the drug review module, update lag, high proportion of invalid warnings, etc., to reduce The risk of iatrogenic drug use and the effect of drug safety guarantee
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
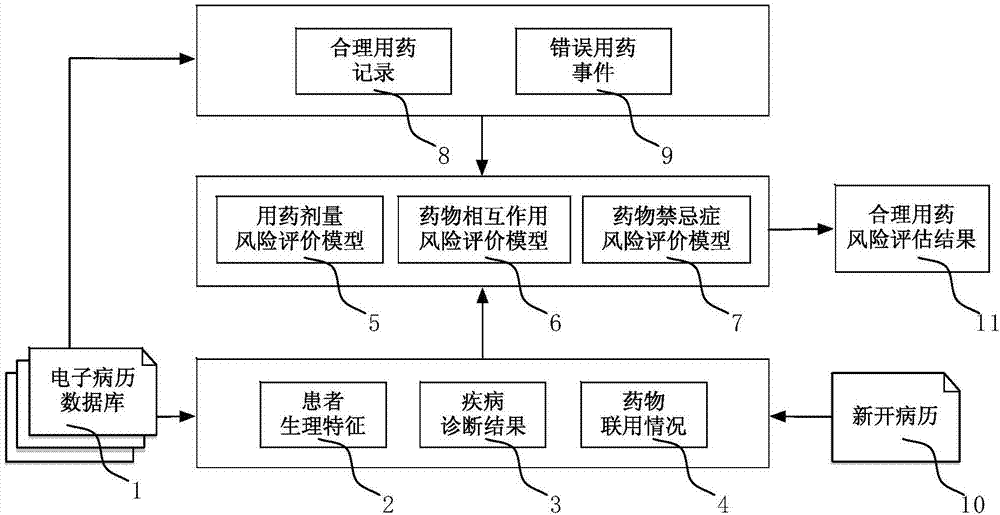
[0030] Such as figure 1 As shown, the electronic medical record database 1 can be obtained through a hospital information system (Hospital Information System, HIS) of a medical institution or other means. In this example, the outpatient electronic medical records database of a tertiary hospital in 2015 was used for one year. After the data were denoised and cleaned, about 3 million valid electronic medical records were obtained.
[0031] The clinical rational drug use risk assessment method based on the big data of electronic medical records includes the following steps:
[0032] S1. Extract rational drug use records and wrong drug use events from the electronic medical record database, as well as corresponding patient physiological characteristics, disease diagnosis results, drug combination and other information, and establish risks related to drug dosage, drug interactions, drug contraindications, etc. Evaluate the model.
[0033] S2. For the newly issued medical records,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com