Synthesis method of daptomycin
A synthesis method and technology of daptomycin are applied in the field of daptomycin synthesis of solid-liquid phase combination, which can solve the problem of high synthesis cost and achieve the desired effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
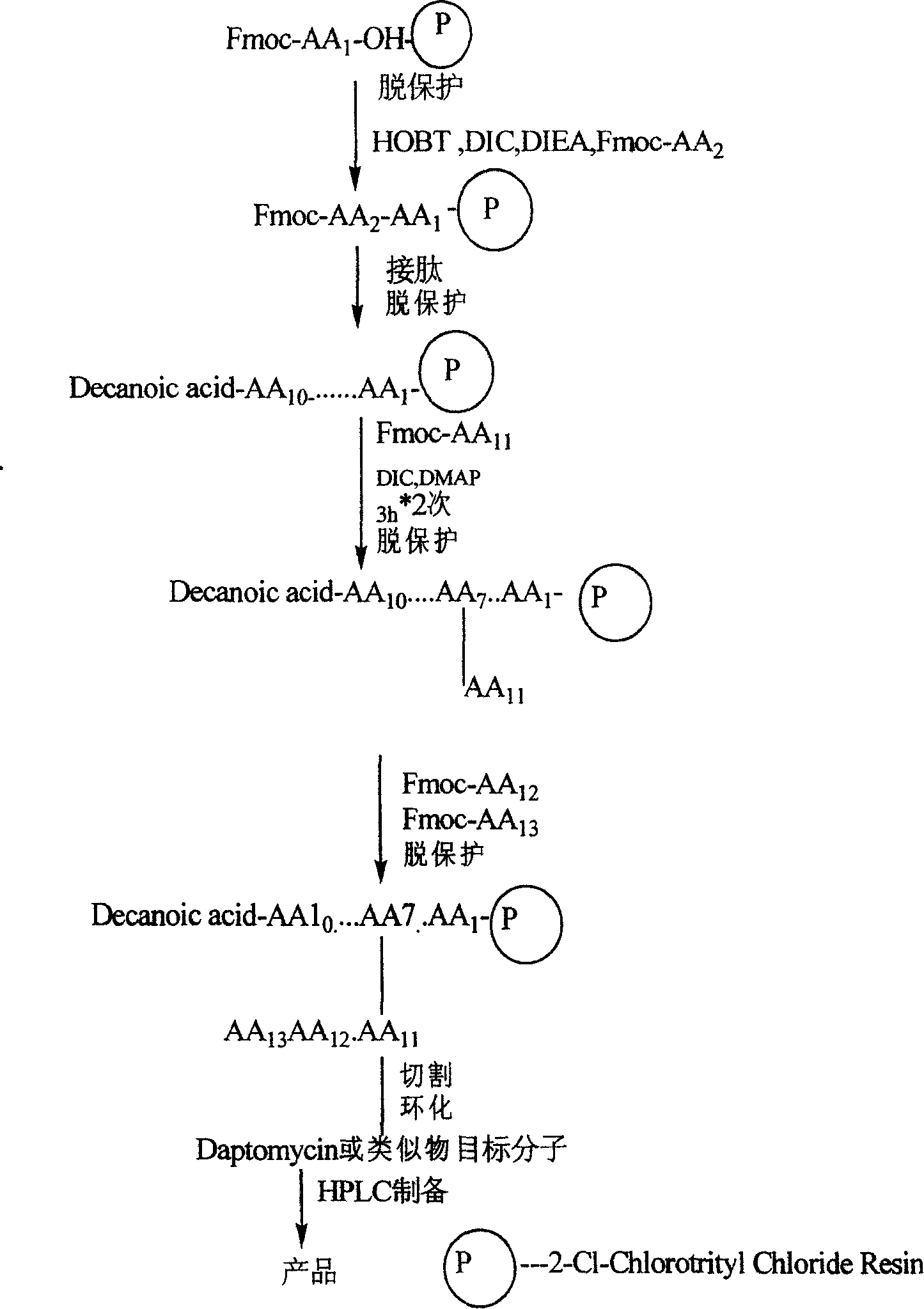
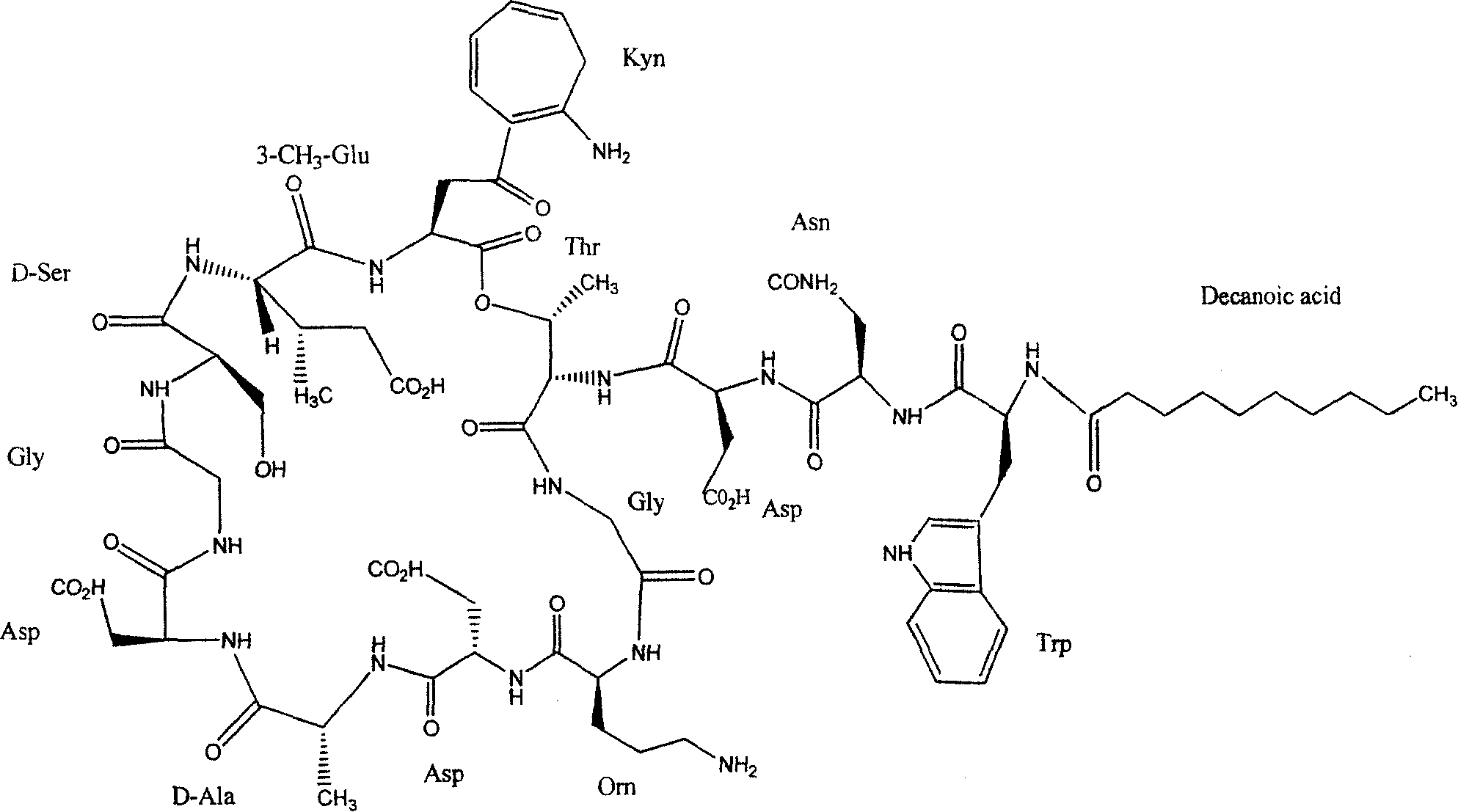
[0048] Synthesis of Daptomycin
[0049]
[0050] 1.1 Solid-phase preparation of fully protected peptides
[0051] Weigh 0.5 g of 2-Cl-Chlorotrityl Chloride resin (0.4-0.5 mmol / g), soak it in 100 ml of DMF to fully swell the resin, and drain it. Take 0.15g of Fmoc-Gly-OH and put it into a peptide bottle, dissolve it in 60ml of DMF, add 0.6ml of DIEA, and react at 25°C for 1 hour. Drained and added 60ml of methanol to react for 10min. Drained, respectively with DMF, MeOH, CH 2 Cl2 and Et 2 O were washed three times each and dried. Weighed 0.081g. The degree of substitution was 0.54 mmol / g.
[0052] Put the above resin into a peptide bottle, add 50ml of 20% piperidine (DMF), shake for 5min, and drain. Then add 50ml of 20% piperidine (DMF) and shake for 15min, then suck dry, wash with DMF, MeOH and CH 2 Cl 2 Each was washed three times in turn, and then followed by Fmoc-Asp(O t Bu)-OH, Fmoc-D-Ala-OH, Fmoc-Asp(O t Bu)-OH, Fmoc-Orn(Boc)-OH, Fmoc-Gly-OH, Fmoc-Thr-OH, Fm...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com