Method for separating and purifying daptomycin
A technology for separation and purification of daptomycin, applied in the field of biomedicine, can solve problems such as complex operation, achieve the effect of reducing side effects and excellent raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
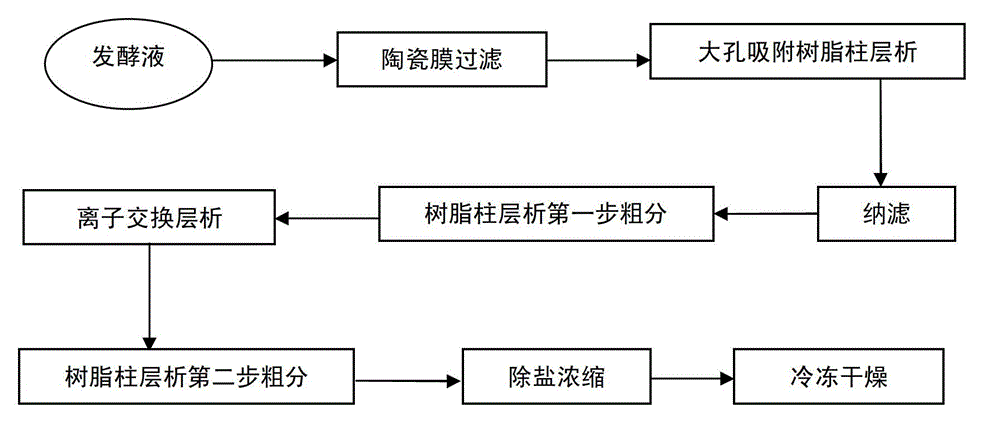
[0042] Receive 20L of fermentation broth to be put into the tank, the fermentation titer is 1200mg / L, and dilute the fermentation broth with 18L of purified water. After dilution, the fermentation unit is 631.5mg / L, the feed liquid temperature is 25°C, and the ceramic membrane is filtered. When concentrated to 6L, add 2.5L of purified water to the non-permeated liquid of the ceramic membrane, stir for 5min, and continue to filter through the ceramic membrane. When concentrated to 6L again, add 2.5L of purified water to the ceramic membrane impermeate, stir for 5min, and continue ceramic membrane filtration. This operation was repeated 8 times, and a total of 20 L of purified water was added. Sampling, HPLC detection of daptomycin 48mg / L in ceramic membrane permeate liquid, ceramic membrane filtration ends. A total of 52 L of ceramic membrane permeate was collected, containing 21.8 g of daptomycin.
[0043]Load the collected ceramic membrane permeate into a macroporous adsorp...
Embodiment 2
[0051] Receive 25L of fermentation broth to be put into the tank, the fermentation titer is 1320mg / L, and dilute the fermentation broth with 25L of 5% ethanol. After dilution, the fermentation unit is 660mg / L, the temperature of the feed liquid is 25°C, and the ceramic membrane is filtered. When concentrated to 8L, add 3L of 5% ethanol to the non-permeated liquid of the ceramic membrane, stir for 5min, and continue to filter through the ceramic membrane. When concentrated again to 8L, add 3L of 5% ethanol to the impermeate liquid of the ceramic membrane, stir for 5min, and continue to filter through the ceramic membrane. This operation was repeated 8 times, and a total of 24 L of 5% ethanol was added. Sampling, HPLC detection of daptomycin 42mg / L in the ceramic membrane permeate, ceramic membrane filtration ends. A total of 65 L of ceramic membrane permeate was collected, containing 30.1 g of daptomycin.
[0052] The collected ceramic membrane permeate was applied to a macro...
Embodiment 3
[0060] Receive 27L of fermentation broth to be put into the tank, the fermentation titer is 1180mg / L, and dilute the fermentation broth with 15L of 30% ethanol. After dilution, the fermentation unit is 758mg / L, the temperature of the feed liquid is 40°C, and the ceramic membrane is filtered. When concentrated to 9L, add 3L of 30% ethanol to the non-permeate liquid of the ceramic membrane, stir for 5min, and continue to filter through the ceramic membrane. When concentrated again to 9L, add 3L of 30% ethanol to the impermeate liquid of the ceramic membrane, stir for 5min, and continue to filter through the ceramic membrane. This operation was repeated 8 times, and a total of 24 L of 30% ethanol was added. Sampling, HPLC detection of daptomycin 40mg / L in the ceramic membrane permeate, ceramic membrane filtration is completed. A total of 56 L of ceramic membrane permeate was collected, containing 28.8 g of daptomycin.
[0061] Load the collected ceramic membrane permeate into a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com