Daptomycin analog and full solid phase synthesis preparation method thereof
A technology of daptomycin and analogues, applied in the field of all-solid-phase synthesis, can solve the problems of cumbersome steps, high cost, energy and time consumption, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
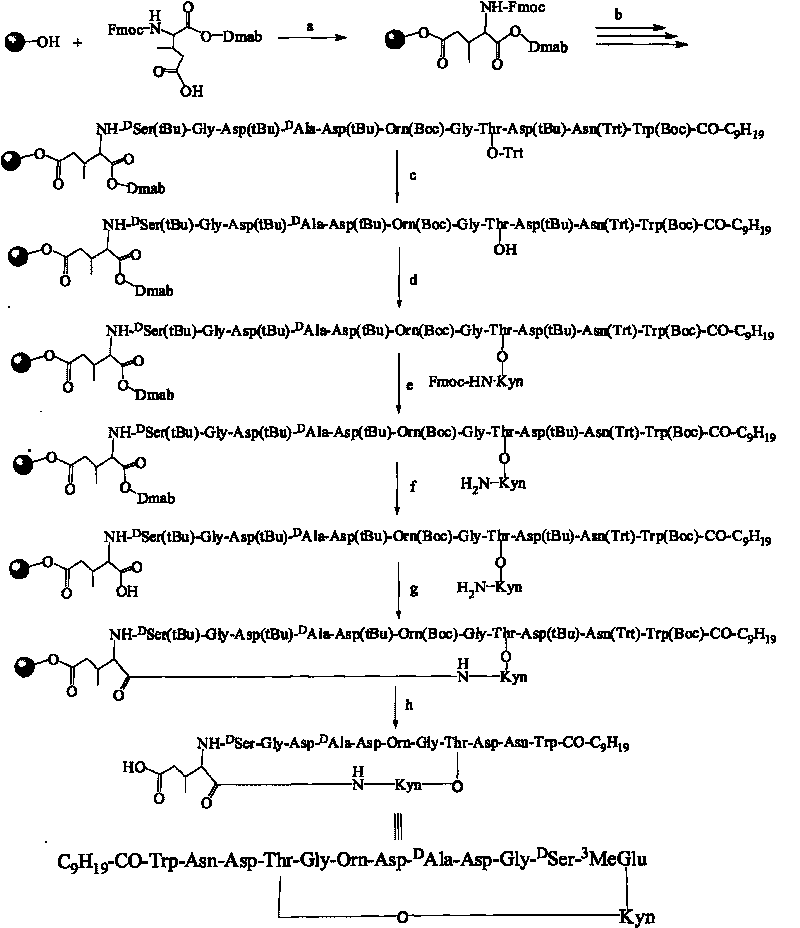
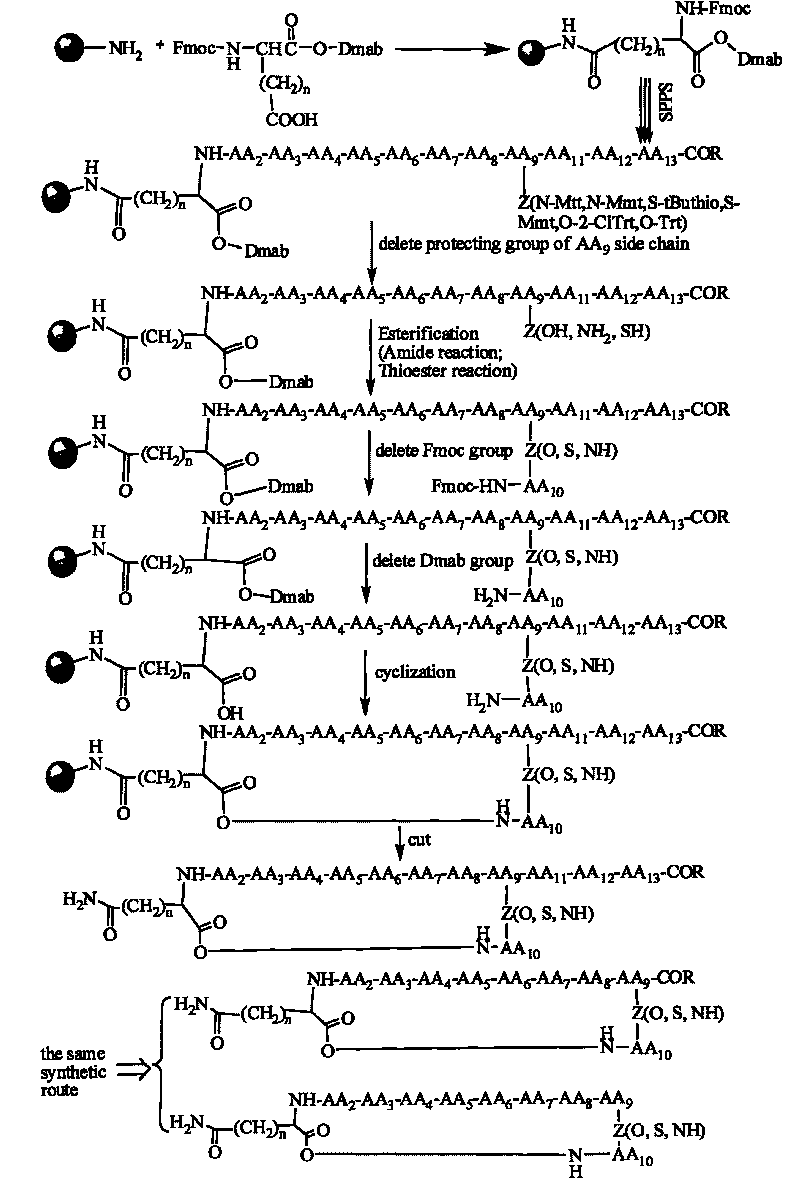
[0086] Now in conjunction with embodiment, accompanying drawing, the present invention will be further described:
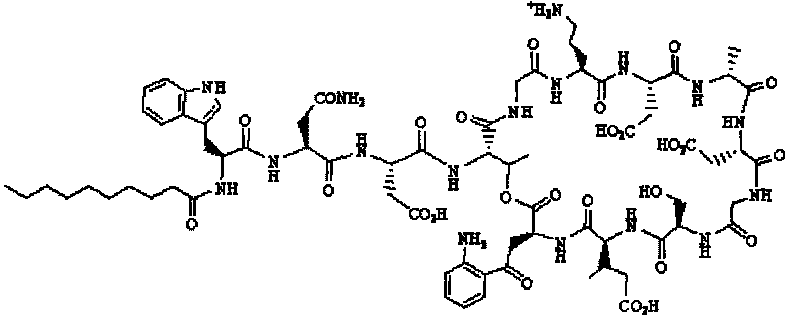
[0087] Example of Synthetic Daptomycin Analogs (1): AA 1 Select Fmoc-Glu-ODmab, AA 9 Choose Fmoc-L-Ser(Trt)-OH, choose C for R 10 h 20 o 2 (n-capric acid). The sequence is:
[0088]
[0089] Its structure diagram is shown in the appendix of the manual Figure 4
[0090] Concrete synthetic steps:
[0091] 1. Prepare the solution
[0092] 1) Remove Fmoc protecting group (Deprotection) solution
[0093] 20% piperidine in DMF, V / V
[0094] 2) Kaiser Test solution
[0095] A.5% ninhydrin ethanol solution, W / V
[0096] B. 80% phenol ethanol solution, W / V
[0097] C.2% 1mmol / L potassium cyanide pyridine solution, V / V
[0098] 2. Resin activation
[0099] Accurately weigh 100mg Rink Amide-AM Resin (0.28mmol / g, 100-200mesh) into a 2mL Bio Spin empty column, add 1mL chloroform (CHCl 3 ) to fully mix, place on a rotary mixer and soak for more than 30 minu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com