Method for synthesizing tetrapeptide isomers by using solid phase peptide synthesis method and applications of tetrapeptide isomers
A technology for solid-phase peptide synthesis and isomerization, applied in peptide preparation methods, tetrapeptide components, chemical instruments and methods, etc., can solve problems such as concentration drop and limitation of biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] The synthetic method of embodiment 1 tetrapeptide isomers
[0081] The synthesis process of tetrapeptide isomer ① (N-Acetyl-Ser-(d)Asp-Lys-Pro-COOH) starting from 5g of dichloro resin as an example:
[0082] 1. Synthesis of Peptide Resin
[0083] (1) Preparation of Fmoc-Pro-resin:
[0084] Weigh 5 grams of 2-chloro-tritylchloromethyl resin, soak it with 100ml of dichloromethane (DCM) for 60 minutes, add 4.5ml of DIEA, 3.38g of Fmoc-Pro-OH to the above resin, and react at 25°C for 2 hour, then add blocking reagent methanol 1.5ml, react for 2 hours at 25°C, wash the resin twice with 35ml isopropanol, and then wash twice with 35ml N,N-dimethylformamide (DMF) to obtain Fmoc-Pro- resin;
[0085] (2) Preparation of Fmoc-Lys(Boc)-OH resin:
[0086] In the Fmoc-Pro-resin of step (1), add 35ml of decapping reagent, react at 25°C for 10 minutes, dry it with a vacuum pump, add 35ml of decapping reagent at 25°C for 30 minutes, dry it, wash with 35ml of isopropanol for 2 Wash t...
Embodiment 2
[0105] The synthetic method of embodiment 2 tetrapeptide isomers (unblocked)
[0106] The synthetic process of tetrapeptide isomer ① (N-Acetyl-Ser-(d)-Asp-Lys-Pro-COOH) starting from 5g of dichloro resin as an example:
[0107] Compared with Example 1, the difference of this example is that no blocking reagent is added in Step 1, and the step of preparing Fmoc-Pro-resin in Step 1 is specifically:
[0108] Weigh 5 grams of 2-chloro-tritylchloromethyl resin, soak it with 100ml of dichloromethane (DCM) for 60 minutes, add 4.5ml of DIEA, 3.38g of Fmoc-Pro-OH to the above resin, and react at 25°C for 2 Hour, resin washes twice with 35ml isopropanol, then washes twice with 35ml N, N-dimethylformamide (DMF), obtains Fmoc-Pro-resin;
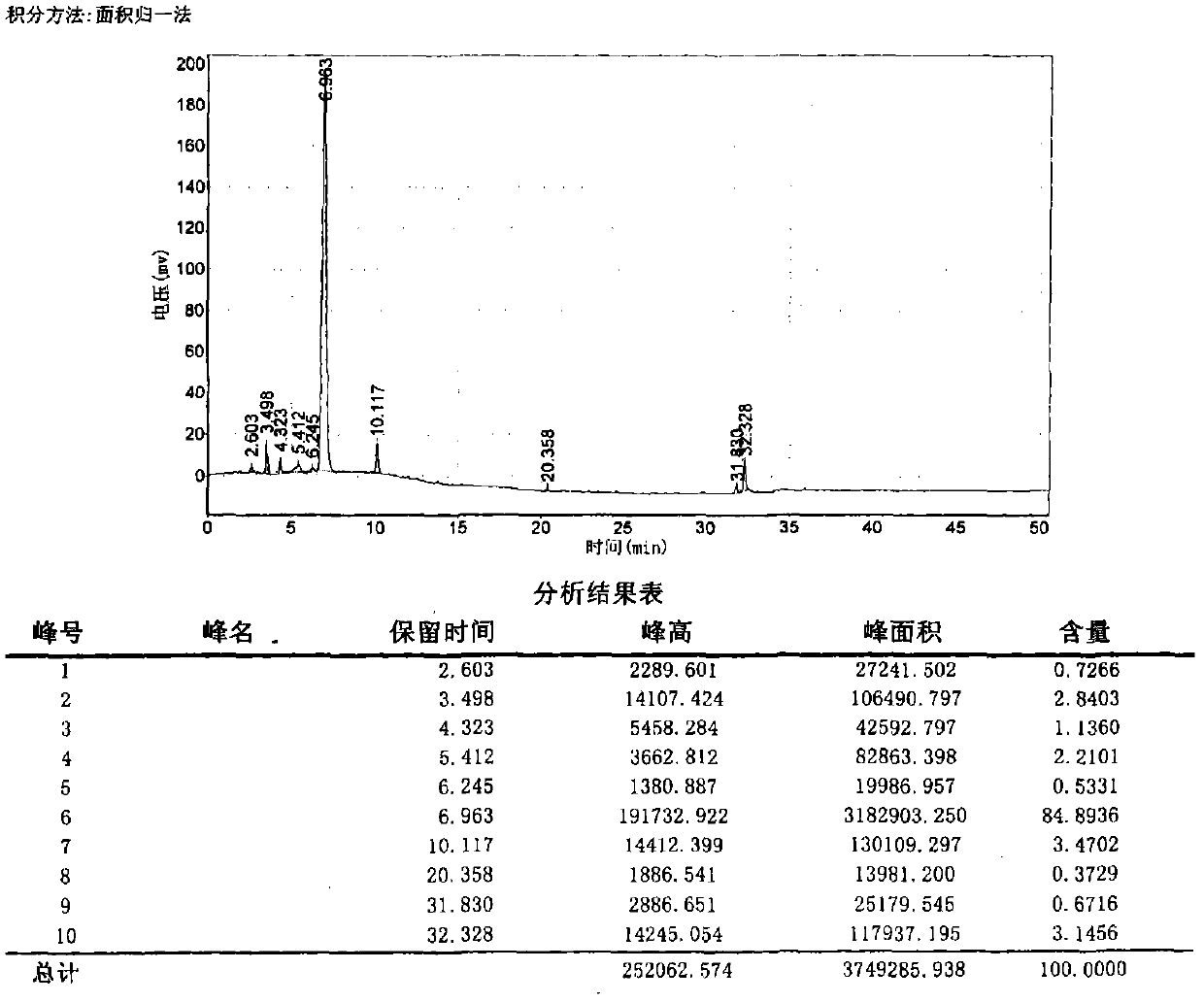
[0109] In this example, N-Acetyl-Ser-(d)Asp-Lys-Pro was synthesized by an unblocked method, the purity of the crude product was 84.8936%, and the purity of the pure product was 98% (HPLC normalization method). The rate is 61.7%. Crude product chromato...
Embodiment 3
[0110] Embodiment 3 The synthetic method of tetrapeptide isomer 2.
[0111] The synthesis process of tetrapeptide isomer ② (N-Acetyl-Ser-Asp-d-Lys-Pro-COOH) starting from 5g of dichloro resin as an example:
[0112] 1. Synthesis of Peptide Resin
[0113] (1) Preparation of Fmoc-Pro-resin:
[0114] Weigh 5 grams of 2-chloro-tritylchloromethyl resin, soak it with 100ml of dichloromethane (DCM) for 60 minutes, add 4.5ml of DIEA, 3.38g of Fmoc-Pro-OH to the above resin, and react at 25°C for 2 hour, then add blocking reagent methanol 1.5ml, react for 2 hours at 25°C, wash the resin twice with 35ml isopropanol, and then wash twice with 35ml N,N-dimethylformamide (DMF) to obtain Fmoc-Pro- resin;
[0115] (2) Preparation of Fmoc-D-Lys(Boc)-OH resin:
[0116] In the Fmoc-Pro-resin of step (1), add 35ml of decapping reagent, react at 25°C for 10 minutes, dry it with a vacuum pump, add 35ml of decapping reagent at 25°C for 30 minutes, dry it, wash with 35ml of isopropanol for 2 Was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com