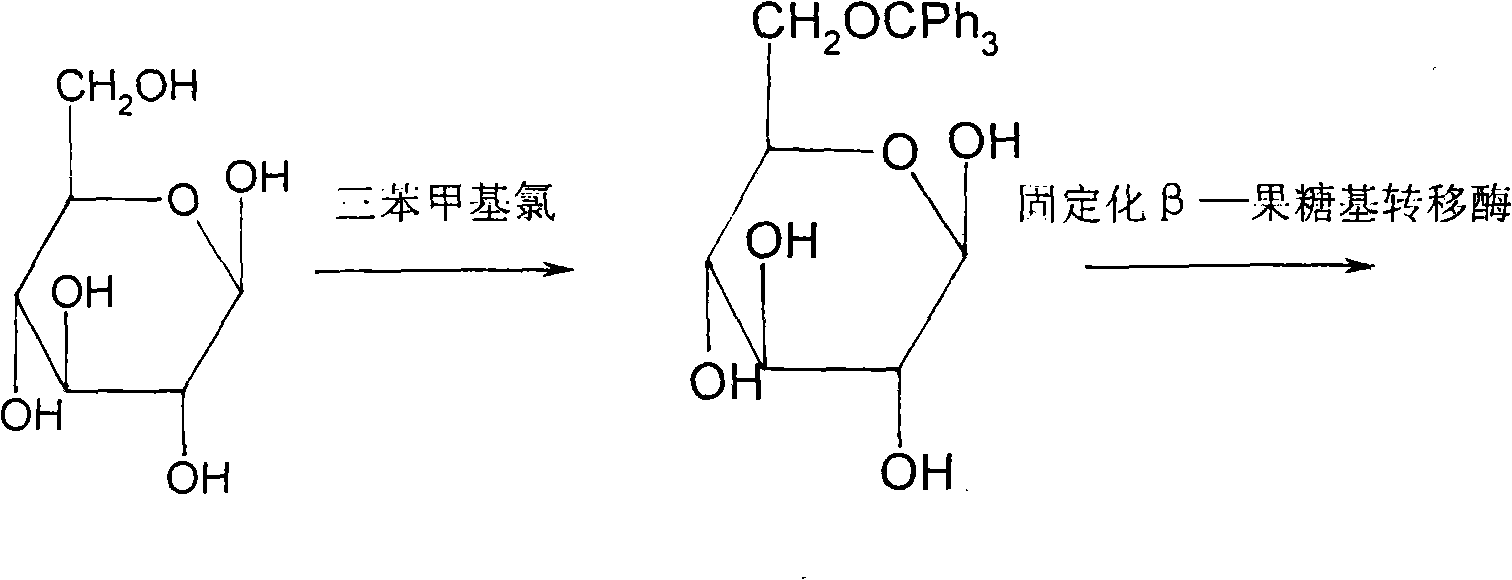
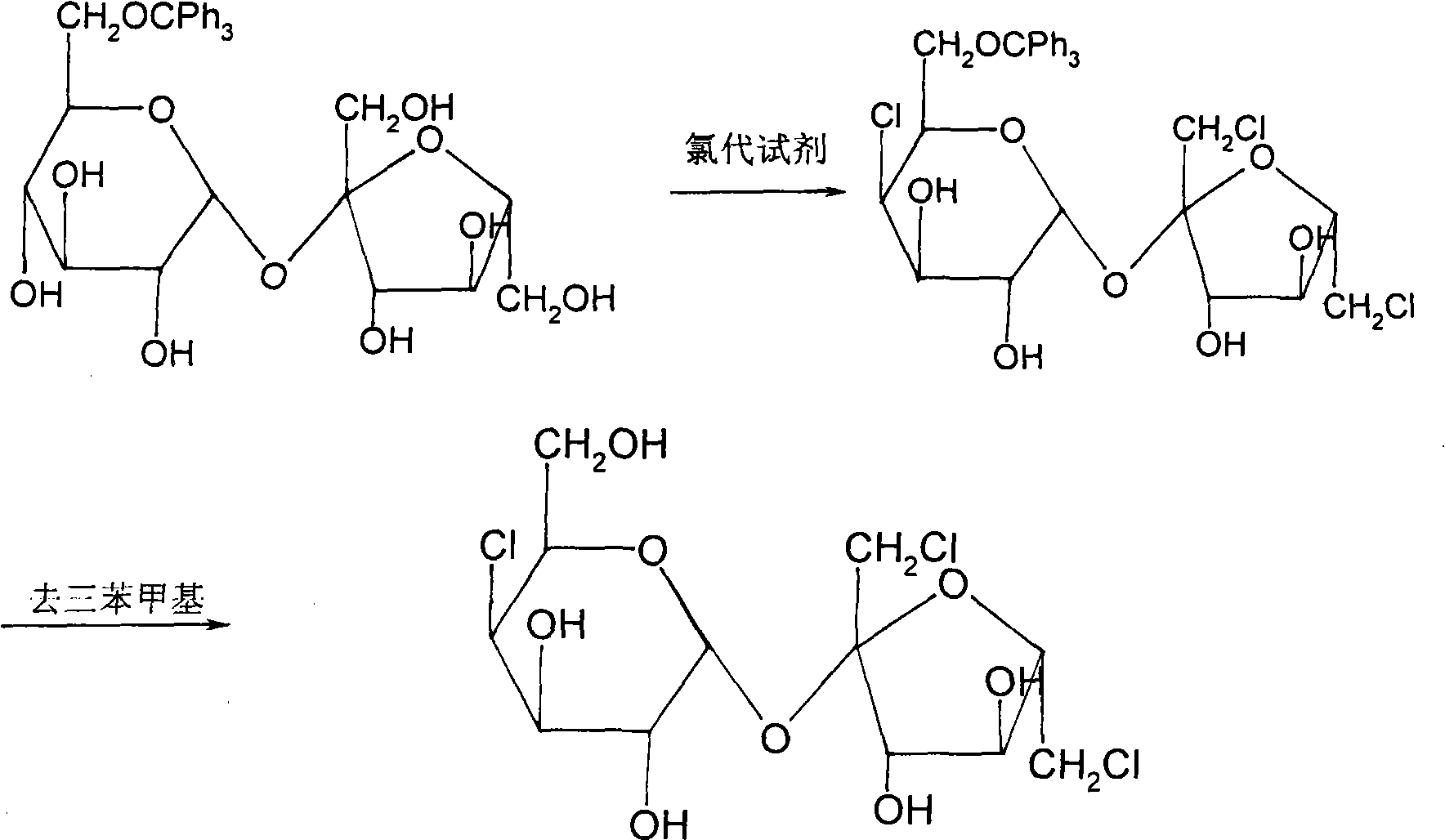
Method for synthesizing sucralose
A technology for sucralose and a synthesis method, which is applied in chemical instruments and methods, sugar derivatives, sugar derivatives, etc., can solve problems such as being unsuitable for industrial production, difficult to separate by double enzyme-chemical method, etc., and improve the utilization rate of materials. , Guaranteed utilization, compact and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 100g of glucose and 300ml of DMF to a 1000ml three-necked flask and stir to dissolve the glucose completely, add 80g of N-methylmorpholine at 40°C, and gradually add 110g of trityl within 1 hour 200ml of DMF solution made of chlorine was reacted at a constant temperature of 40°C for 4 hours, then 37.5g of sodium bicarbonate was added for neutralization, and water, N-methylmorpholine, and DMF were distilled off under vacuum conditions. The reaction concentrate was dried to obtain 188.3 g of 6-O-trityl glucose with a yield of 80.8% and a purity of 81% by HPLC.
Embodiment 2
[0036] Add 100g of glucose and 300ml of DMF to a 1000ml three-necked bottle and stir to dissolve the glucose completely, add 80g of N-methylmorpholine at 30°C, and gradually add 92g of trityl within 1 hour 200ml of DMF solution made of chlorine was reacted at a constant temperature of 30°C for 8 hours, then 37.5g of sodium bicarbonate was added for neutralization, and water, N-methylmorpholine, and DMF were distilled off under vacuum conditions. The reaction concentrate was dried to obtain 145.6 g of 6-O-trityl glucose with a yield of 62.5% and a purity of 78% by HPLC.
Embodiment 3
[0038] Add 100g of glucose and 300ml of DMF to a 1000ml three-necked bottle and stir to dissolve the glucose completely, add 80g of N-methylmorpholine at 30°C, and gradually add 138g of trityl within 1 hour 200ml of DMF solution made of chlorine was reacted at a constant temperature of 30°C for 8 hours, then 37.5g of sodium bicarbonate was added for neutralization, and water, N-methylmorpholine, and DMF were distilled off under vacuum conditions. The reaction concentrate was dried to obtain 202.7 g of 6-O-trityl glucose, the yield was 86.9%, and the purity by HPLC was 75%.
[0039] 2. Generation of 6-O-trityl sucrose (product II)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com