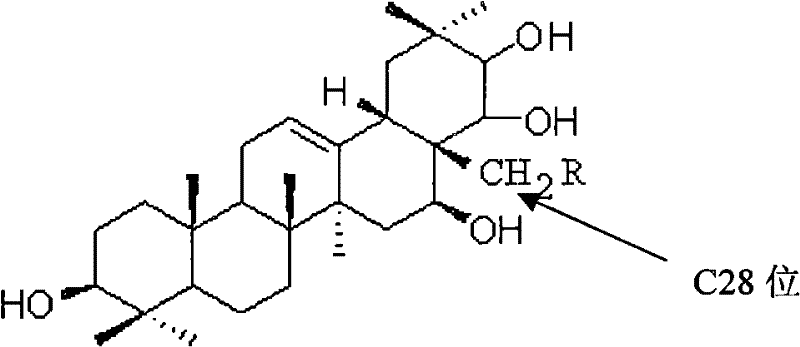
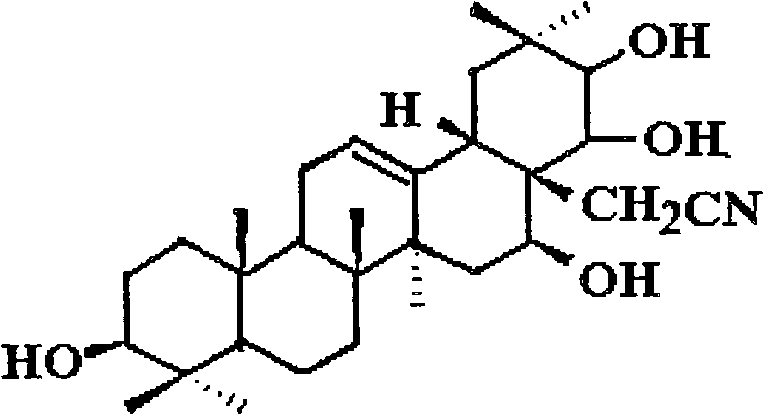
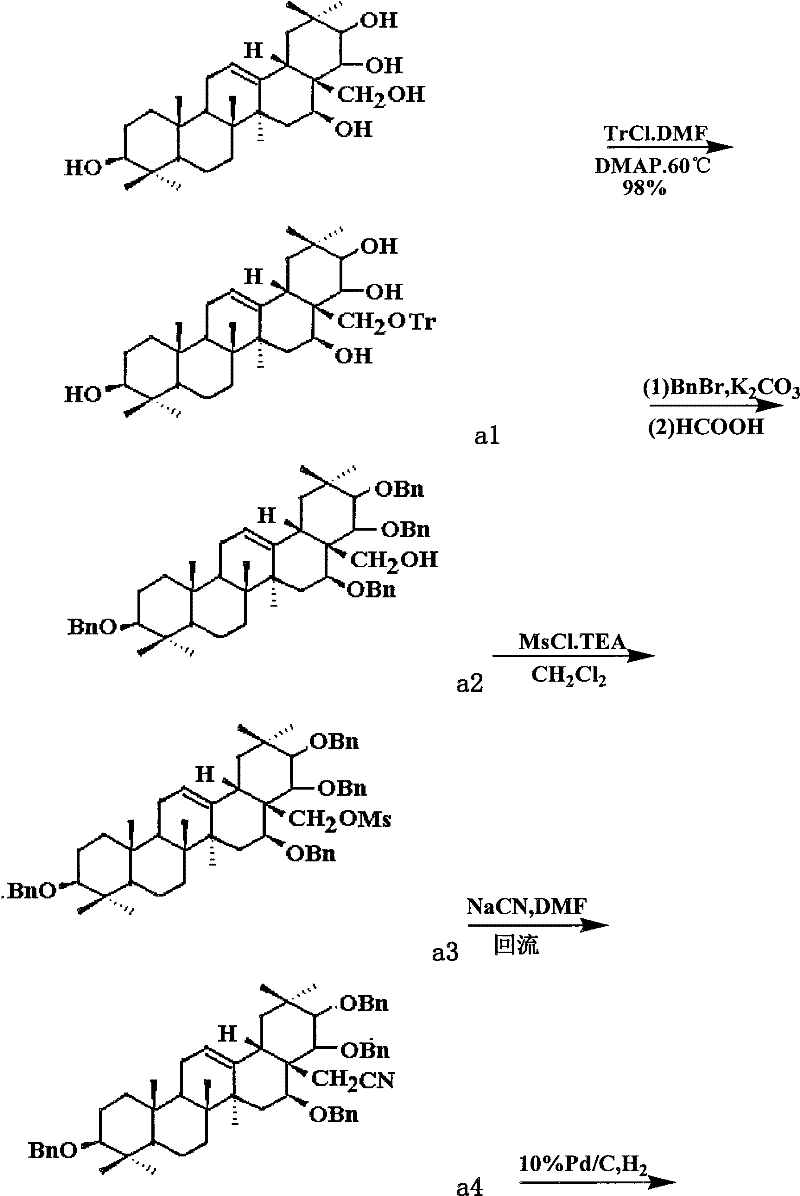
Theasapogenol derivative with anti-tumour activity and preparing method and application thereof
A technology of anti-tumor activity and thea-sapogenin, which is applied in the field of medicine, can solve the problems that the anti-tumor activity of thea-sapogenin and its derivatives has not been reported, and achieve the effect of convenient industrial production, high product purity and high anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] (1) Get tea presapogenol-A crystal (5g, about 8.9mmol) and 50mlDMF (dimethylformamide), stir and dissolve; add triphenylchloromethane (2.5g) reaction under the protection of argon, Then slowly add DMAP (1.2 g about 10 mmol) to catalyze the reaction, and react at 60° C. for 2 hours. After the reaction was completed, the mixture was diluted with water, and ethyl acetate was added for extraction. The organic phase was dried with anhydrous magnesium sulfate, filtered, and distilled under reduced pressure to obtain the crude product a1 with a conversion rate of 98%. The crude product was used in the next reaction without purification.
[0031] (2) Mix a1 (7.0g, about 8.7mmol), tetrabutylammonium iodide (0.4g, 1.1mmol) and tetrahydrofuran (50 mL), and add sodium hydroxide (0.4g, 17.4mmol) under stirring. After about 20 minutes, benzyl bromide (44 mL about 35 mmol) was added to the reaction system, and heated to reflux for 2 h. The reaction mixture was distilled off under r...
Embodiment 2
[0042] (1) Mix 2g (about 2.3mmol) of a2 and 20ml of 1.2-dichloromethane, stir to dissolve, add 0.2ml (0.0029mol) of azide in benzene, cool to -15°C in an ice-salt bath, stir and drop Add a mixed solution containing 0.5 ml of boron trifluoride in ether and 0.5 ml of dichloromethane, keeping the reaction temperature below -15°C. After the dropwise addition, the reaction was stirred at -15°C for 1 hour, and the reaction was checked by TLC for completeness. Add 0.2ml of pyridine to decompose unreacted boron trifluoride, a precipitate is formed, add 10ml of water and stir to dissolve the precipitate, separate the organic layer, wash with 5ml of 5% hydrochloric acid solution and water (10ml×3), anhydrous sodium sulfate After drying, the solvent was evaporated to dryness, and recrystallized with about 50 ml of acetone-methanol (1:1) to obtain white crystal b1.
[0043](2) Add b1 to a 250ml autoclave, add 20ml of dry ethyl acetate to dissolve, add 0.3g of 10% palladium / carbon catalys...
Embodiment 3
[0052] (1) Take a2 (2g, about 2.3mmol), 4-carboxy-2,2,6,6-tetramethylpiperidine nitroxide radical (0.1g), dicyclohexylcarbodiimide (0.1g) Add 50ml of dichloromethane to dissolve, electromagnetically stir for 6h, and detect the end point of the reaction by TLC. Concentrate the solvent under reduced pressure, then use petroleum ether: acetone as the developing solvent, and prepare through a thin layer of silica gel GF254 to obtain compound c1.
[0053] (2) After c1 was dissolved in ethanol (100ml), 10% Pd / C (1g) was added, 1 atmosphere of hydrogen gas was introduced, and the mixture was refluxed at room temperature for 12h. The mixture was filtered to remove insoluble matter, and the residue after distilling off ethanol from the filtrate under reduced pressure was extracted with ethyl acetate, and the extract was washed with water and saturated sodium chloride, respectively. The organic phase was separated and dried over anhydrous magnesium sulfate. The desiccant was removed b...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com