A kind of preparation method of folic acid thiolated derivative
A technology for folic acid derivatives and derivatives, which is applied in the field of preparation of folic acid thiolated derivatives, can solve the problems of difficulty in product separation, difficulty in realizing large-scale production, and increasing difficulty in operation, and achieves overcoming low product yield and overcoming product The effect of difficult separation and improved product separation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
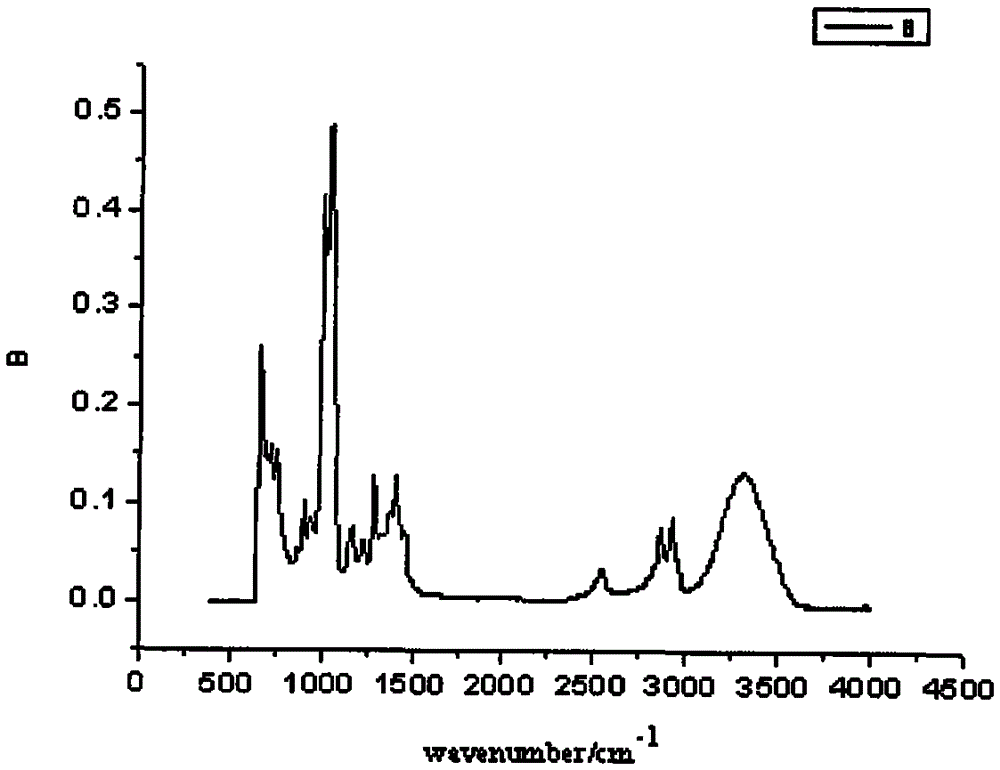
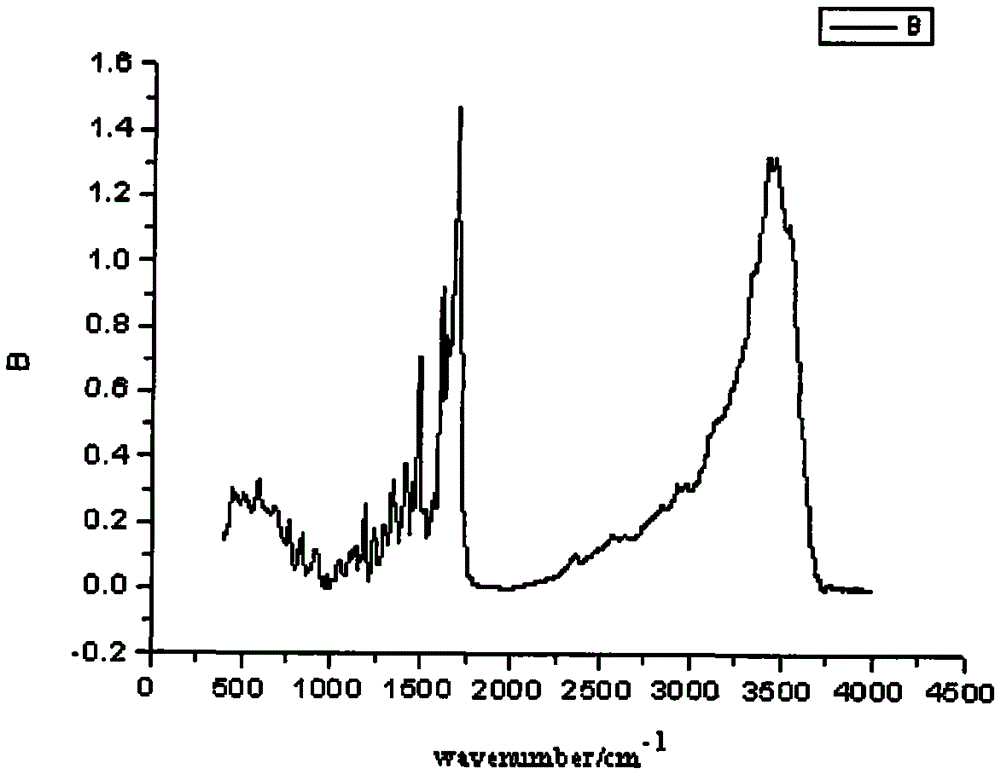
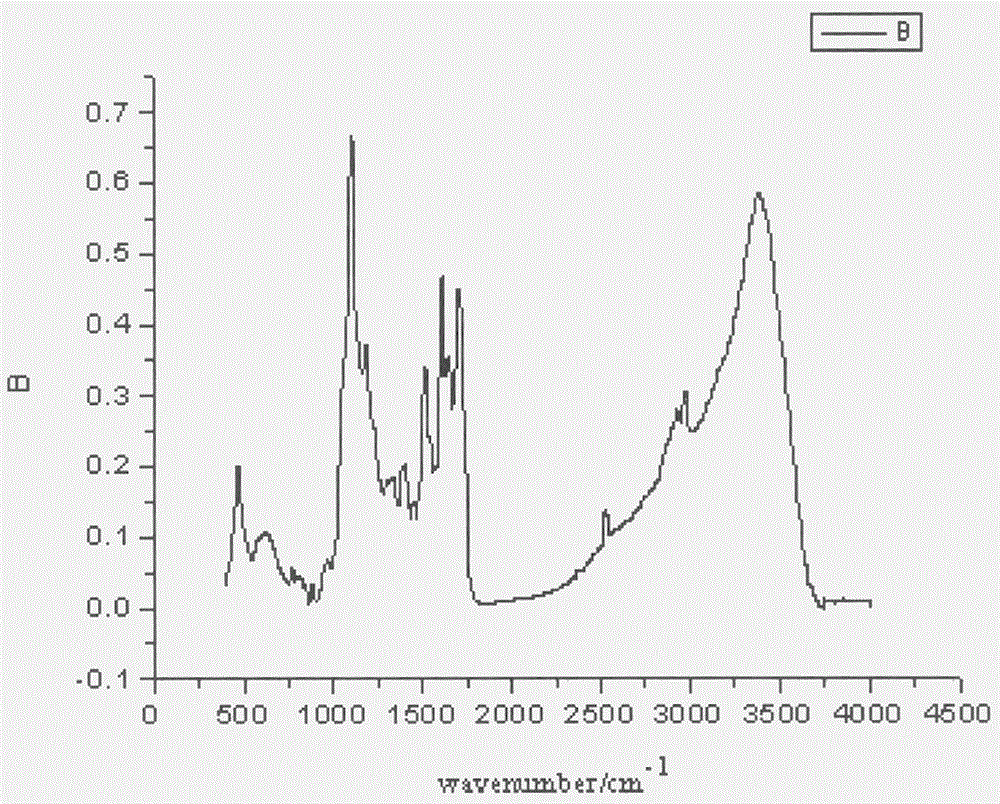
Image
Examples
Embodiment 1
[0042] Protection of Thiolyl in 3-Mercaptopropanol (MP)
[0043] In a 100mL Erlenmeyer flask equipped with a magnet and a constant pressure funnel, add 25mL of tetrahydrofuran solution dissolved with 0.9216g (0.0100mol) 3-mercaptopropanol (MP), and then add 25mL of tetrahydrofuran solution dissolved with 5mmol sodium hydroxide After stirring for 30 minutes, 25 mL of tetrahydrofuran dissolved in 2.6033 g of triphenylmethanol was added, and stirred for 6 hours. The whole process temperature was controlled at 45°C. After the reaction, put the flask containing the mixed solution into a rotary evaporator, set the temperature at 45°C, and stop the rotary evaporation until the solution no longer produces bubbles, then dissolve the product with 20 mL of chloroform, and then extract it three times with 20 mL of distilled water. The organic phase was taken, dried with anhydrous sodium sulfate, and the solvent was distilled off again, and the crude product was recrystallized with aceton...
Embodiment 2
[0045] 2-Mercapto-3-butanol (MB) Thiol Protection
[0046] In a 100mL Erlenmeyer flask equipped with a magnet and a constant pressure funnel, add 25mL of tetrahydrofuran solution dissolved with 1.062g (0.0100mol) 2-mercapto-3-butanol (MB), then add 25mL with 5mmol sodium hydroxide After stirring for 30 minutes, 25 mL of tetrahydrofuran dissolved in 2.6033 g of triphenylmethanol was added, and stirred for 9 hours. The whole process temperature was controlled at 45°C. After the reaction, put the flask containing the mixed solution into a rotary evaporator, set the temperature at 45°C, and stop the rotary evaporator until the solution no longer produces bubbles, then dissolve it with 20mL of chloroform, and then extract it three times with 20mL of distilled water. phase, dried with anhydrous sodium sulfate, evaporated the solvent again, and recrystallized the crude product with acetone to obtain the product 2-tritylthio-3-butanol with a yield of 91%.
Embodiment 3
[0048] Sulfhydryl Protection of 3-Mercaptohexanol (MH)
[0049] In the 100mL Erlenmeyer flask equipped with magneton and constant pressure funnel, add 25mL of tetrahydrofuran solution dissolved with 1.3424g (0.010mol) 3-mercaptohexanol (MH), then add 25mL of tetrahydrofuran solution dissolved with 5mmol sodium hydroxide, After stirring for 30 minutes, 25 mL of tetrahydrofuran dissolved in 2.6033 g of triphenylmethanol was added, and stirred for 12 hours. The whole process temperature was controlled at 45°C. After the reaction, put the flask containing the mixed solution into a rotary evaporator, set the temperature at 45°C, and stop the rotary evaporator until the solution no longer produces bubbles, then dissolve it with 20mL of chloroform, and then extract it three times with 20mL of distilled water. Phase was dried with anhydrous sodium sulfate, and the solvent was distilled off again. The crude product was recrystallized with acetone to obtain the product 3-tritylthiohexa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com