Asymmetric anionic copolymerization method of methacrylate chiral polymer
A technology of methacrylic acid and methacrylamide, which is applied in the synthesis field of functional polymer materials, can solve the problems of less research on copolymerization systems, and achieve the effects of simple operation, easy realization and mature technology.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
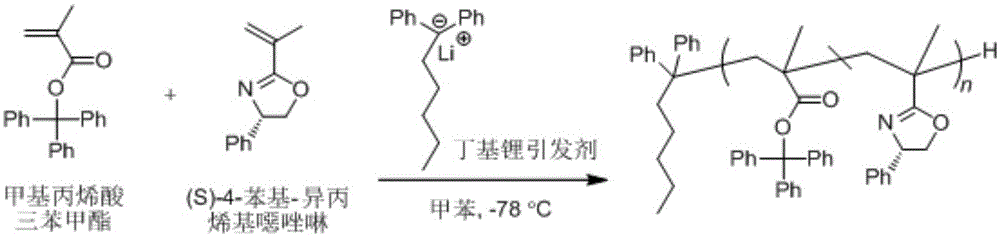
[0029] 1. Take 10.0g trityl chloride and fully dissolve it in toluene at room temperature, then N2 Add methacrylic acid (equal to trityl chloride equivalent) under protection, then drop triethylamine (control the dropping rate at 3 drops / second, 2.3 times the equivalent of trityl chloride), heat up to 80°C, Stir and reflux for 3.5 hours, stop the reaction and lower the temperature, wash with toluene, filter, rotary evaporate, and purify by recrystallization to finally obtain pure trityl methacrylate monomer with a yield of 60%.
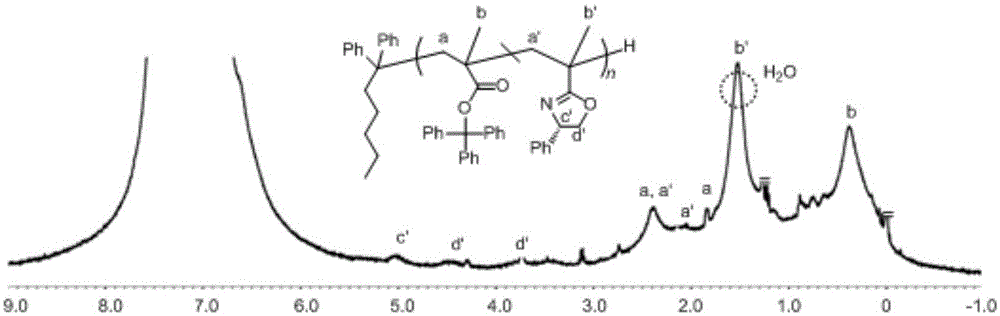
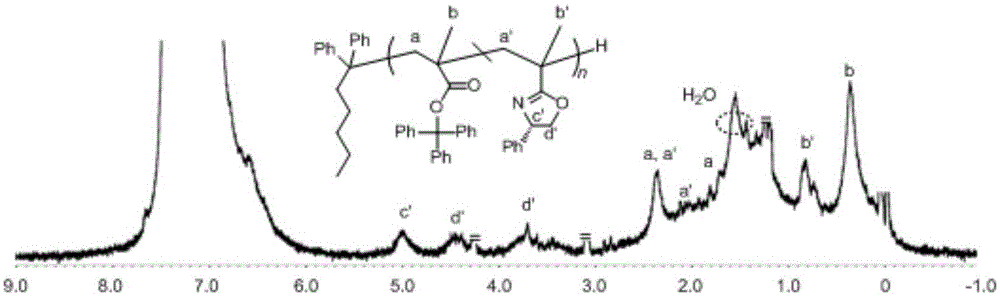
[0030] 2. Add 0.5g of trityl methacrylate monomer into the polymerization tube under the protection of argon, and add 5mL of toluene (10 times the volume of the trityl methacrylate monomer) in the polymerization tube under the protection of argon. , 0.3mL chiral oxazoline monomer (the equivalent ratio with trityl methacrylate monomer is 1:1) and 0.3mL n-butyllithium anion initiator (control drop rate is 3 drops / second, two The ratio of the total amoun...
specific Embodiment approach 2
[0034] 1. Take 10.0g trityl chloride and fully dissolve it in toluene at room temperature, then N 2 Add methacrylic acid (equal to trityl chloride equivalent) under protection, then drop triethylamine (control the dropping rate at 2 drops / second, 2.3 times the equivalent of trityl chloride), heat up to 80°C, Stir and reflux for 4 hours, stop the reaction and lower the temperature, wash with toluene, filter, rotary evaporate, and purify by recrystallization to finally obtain pure trityl methacrylate monomer with a yield of 62%.
[0035] 2. Add 0.5g of trityl methacrylate monomer into the polymerization tube under the protection of argon, and add 5mL of toluene (10 times the volume of the trityl methacrylate monomer) in the polymerization tube under the protection of argon. , 0.3mL chiral oxazoline monomer (the equivalent ratio with trityl methacrylate monomer is 1:1) and 0.3mL n-butyllithium anion initiator (the control rate of addition is 1 drop / second, two The ratio of the t...
specific Embodiment approach 3
[0039] 1. Take 10.0g trityl chloride and fully dissolve it in toluene at room temperature, then N 2 Add methacrylic acid (equal to trityl chloride equivalent) under protection, then drop triethylamine (control the dropping rate at 2 drops / second, 2.3 times the equivalent of trityl chloride), heat up to 80°C, Stir and reflux for 4 hours, stop the reaction and lower the temperature, wash with toluene, filter, rotary evaporate, and purify by recrystallization to finally obtain pure trityl methacrylate monomer with a yield of 67%.
[0040] 2. Add 0.5g of trityl methacrylate monomer into the polymerization tube under the protection of argon, and add 5mL of toluene (10 times the volume of the trityl methacrylate monomer) in the polymerization tube under the protection of argon. , 0.3mL chiral oxazoline monomer (the equivalent ratio with trityl methacrylate monomer is 1:1) and 0.3mL n-butyllithium anion initiator (the control rate of addition is 1 drop / second, two The ratio of the t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com