Theasapogenol derivative with anti-HIV (Human Immunodeficiency Virus) activity, preparation method and application thereof
A technology of tea saponin and derivatives, which is applied in the field of medicine to achieve the effects of high anti-HIV activity, easy control of reaction conditions, and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
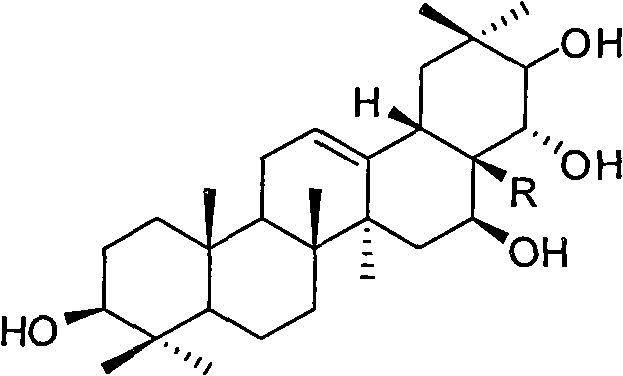
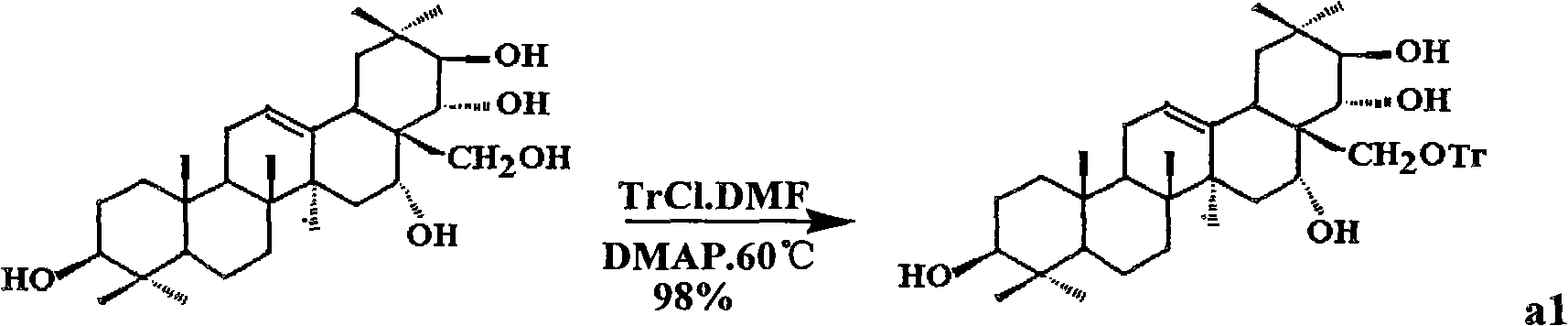
[0032] Embodiment 1: Synthetic compound shown in formula II
[0033] (1) Get tea presapogenol-A (5g, about 8.9mmol) and 50mlDMF (dimethylformamide) and mix, stir and dissolve; add TrCl (trityl chloride 18mmol) reaction under the protection of argon, Then slowly add DMAP (1.2 g about 10 mmol) to catalyze the reaction, and react at 60° C. for 2 hours. After the reaction was completed, the mixture was diluted with water, and ethyl acetate was added for extraction. The organic phase was dried with anhydrous magnesium sulfate, filtered, and distilled under reduced pressure to obtain the crude product a1 with a conversion rate of 98%. The crude product was used in the next reaction without purification.
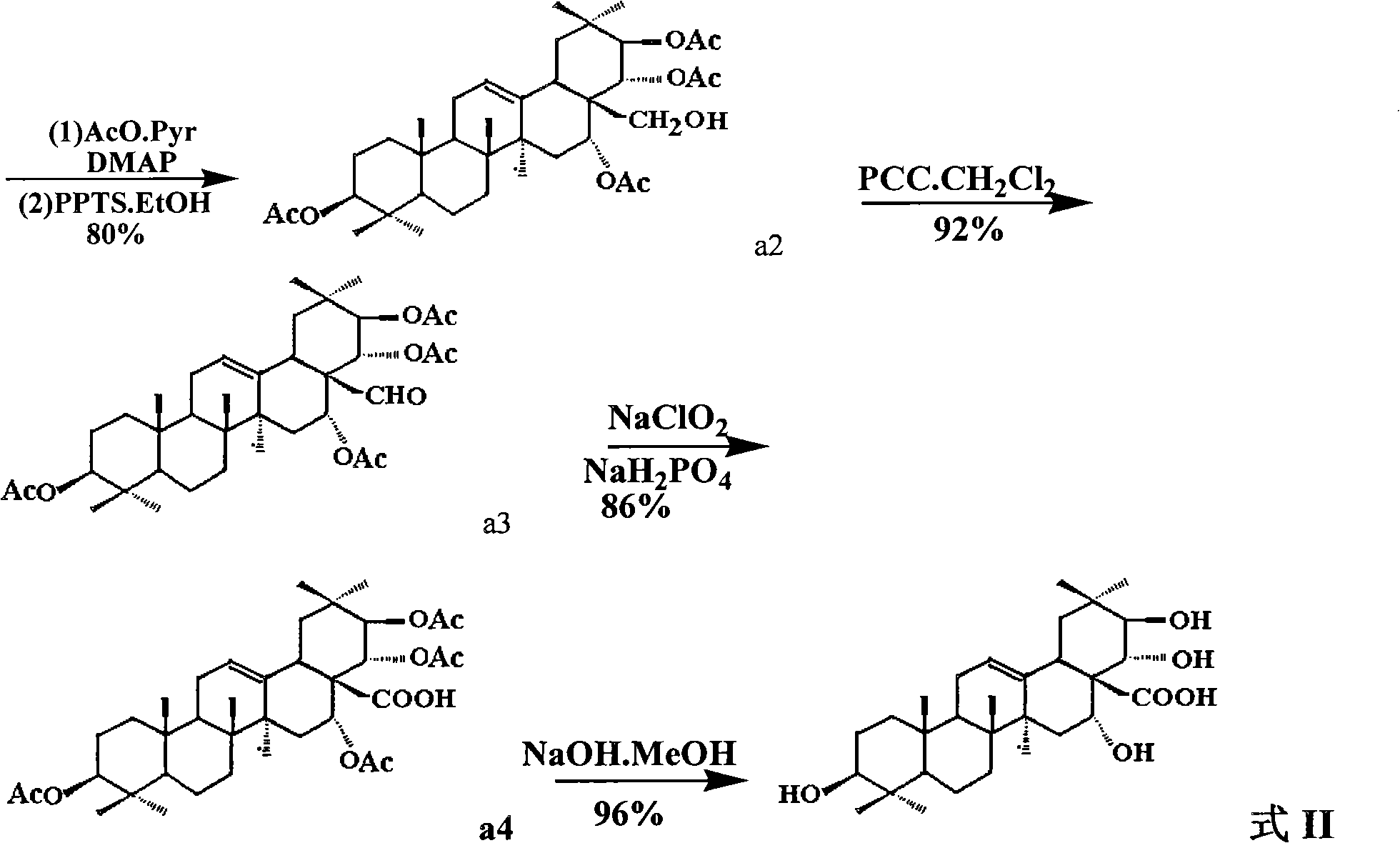
[0034] (2) Dissolve a1 (7.3g, about 8.7mmol) and anhydrous pyridine (50ml), then add 10ml of acetic anhydride, slowly add DMAP (1.2g, about 10mmol), and stir the mixture at room temperature overnight. It was then frozen to 0°C and 1N HCl (25ml) was added. Extracted 3 times with...
Embodiment 2
[0043] Embodiment 2: Synthetic compound shown in formula III
[0044] (1) a4 (1.5g about 2mmol) and anhydrous CH 2 Cl 2(20ml) mixed, stirred to dissolve, added EDC (3mmol) and HOBT (3mmol), then added L-leucine methyl ester (2.5mmol) to the solution, stirred overnight, the solution was washed with CH 2 Cl 2 (50ml) extract, CH 2 Cl 2 Layer was washed three times with water (50ml), washed with anhydrous MgSO 4 Dry, filter to remove anhydrous sodium sulfate, and concentrate under reduced pressure to obtain a crude product, which is purified by silica gel column chromatography, and finally crystallized with ethyl acetate and n-butane to obtain b1 with a yield of 96%.
[0045] (2) Dissolve b1 (1.2g about 1mmol), MeOH (4ml) and THF (4ml), and add 4N NaOH (2ml) to the solution. After the reaction is complete, the mixture is acidified to pH 4 with hydrochloric acid, and then washed with chloroform Extract three times (3×50ml) with anhydrous MgSO 4 Dry, filter to remove anhydrou...
Embodiment 3
[0049] Embodiment 3: Synthesis of compounds shown in formula IV
[0050] (1) a4 (1.5g about 2mmol) and anhydrous CH 2 Cl 2 (20ml) were mixed, stirred to dissolve, EDC (3mmol) and HOBT (3mmol) were added, and then 4S-(8-aminooctylamide)-3R-hydroxyl-6-methylheptanoic acid benzyl ester (1.6 mmol), stirred overnight, the solution with CH 2 Cl 2 (50ml) extract, CH 2 Cl 2 Layer was washed three times with water (50ml), washed with anhydrous MgSO 4 Dry, filter to remove anhydrous sodium sulfate, and concentrate under reduced pressure to obtain a crude product, which is purified by silica gel column chromatography, and finally crystallized with ethyl acetate and n-butane to obtain c1 with a yield of 96%.
[0051] (2) Dissolve c1 (0.5g about 1mmol), MeOH (4ml) and THF (4ml), and add 4N NaOH (2ml) to the solution. After the reaction is complete, the mixture is acidified to pH 4 with hydrochloric acid, and then washed with chloroform Extract three times (3×50ml) with anhydrous MgS...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com