Synthesis method of bagasse xylan gallic acid trimesic acid diesterified derivative
A technology of gallate and benzenetricarboxylic acid, which is applied in the field of polymer materials, can solve the problems of limited anti-biological activity, and achieve the effect of easy control of process conditions and high biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
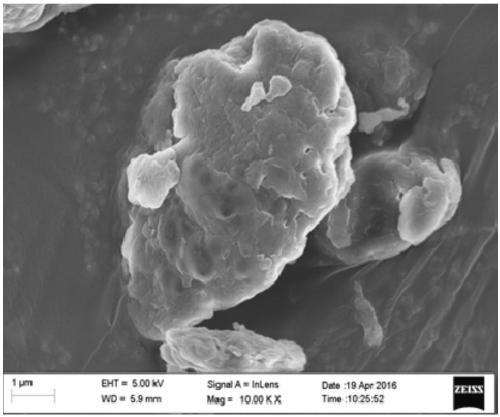
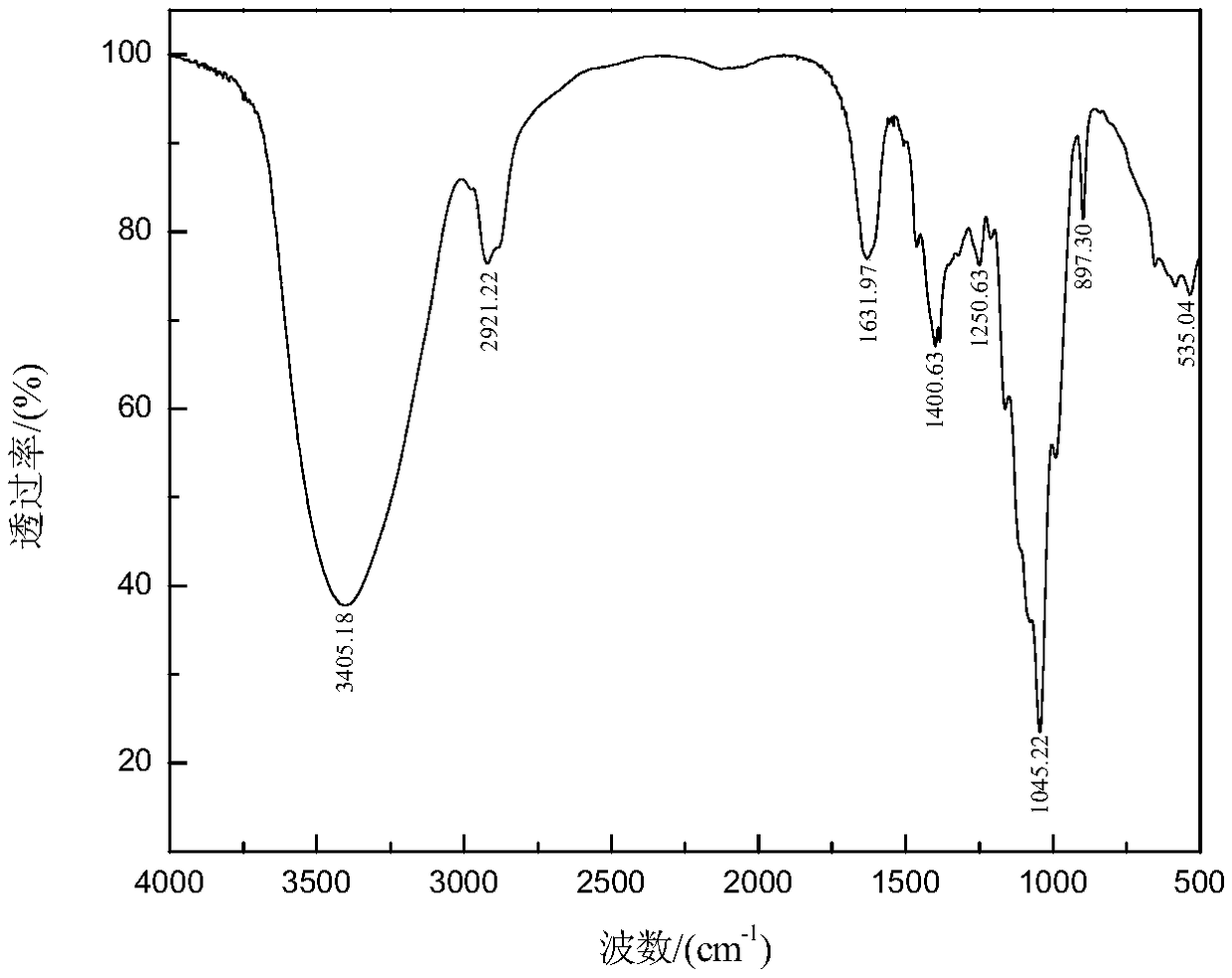
Image
Examples
Embodiment
[0046](1) Weigh 10 g of gallic acid and add it to a 250 mL four-necked flask, and add 20 mL of analytically pure acetic anhydride and 13 mL of analytically pure pyridine to it, control the temperature of the ice bath at 20° C., and react for 8 hours under stirring. Add 22mL of hydrochloric acid solution with a mass fraction of 20%, stir evenly, and precipitate a white precipitate of crude triacetylgallic acid.
[0047] (2) The crude product of triacetyl gallic acid obtained in the suction filtration step (1), and the precipitate was fully washed with 10 mL of distilled water for 3 times and then suction filtered, and the filter cake was sent to a 50° C. vacuum constant temperature drying oven for drying for 24 hours to constant weight to obtain triacetyl gallnut acid.
[0048] (3) Weigh 6g of triacetyl gallic acid obtained in step (2) into a 250mL four-neck flask, and add 30mL of analytically pure cyclohexane and 20mL of analytically pure N,N-dimethylformamide into it, and sti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com