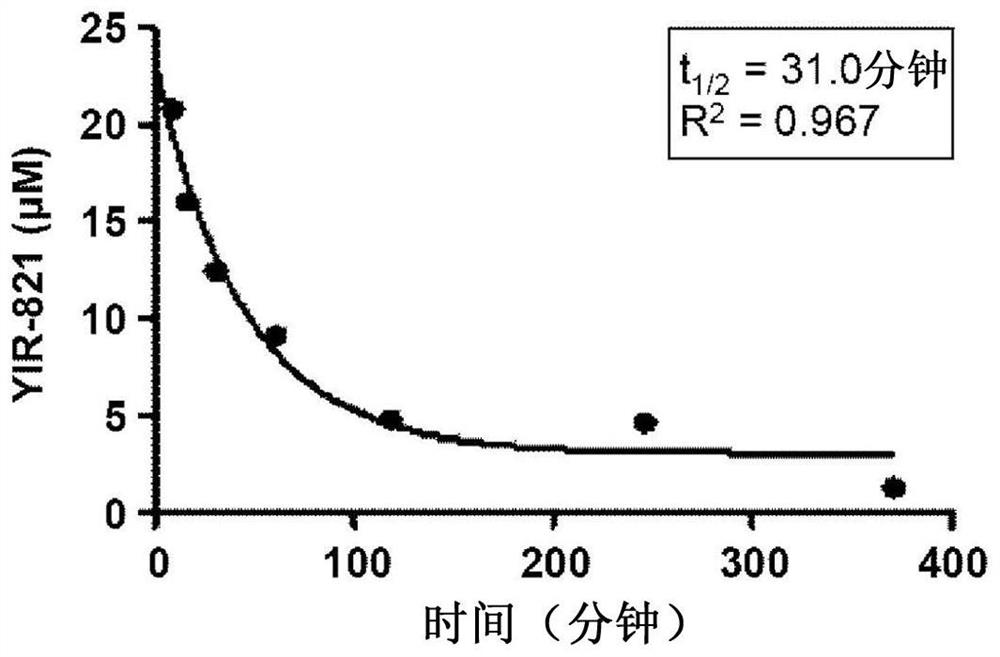
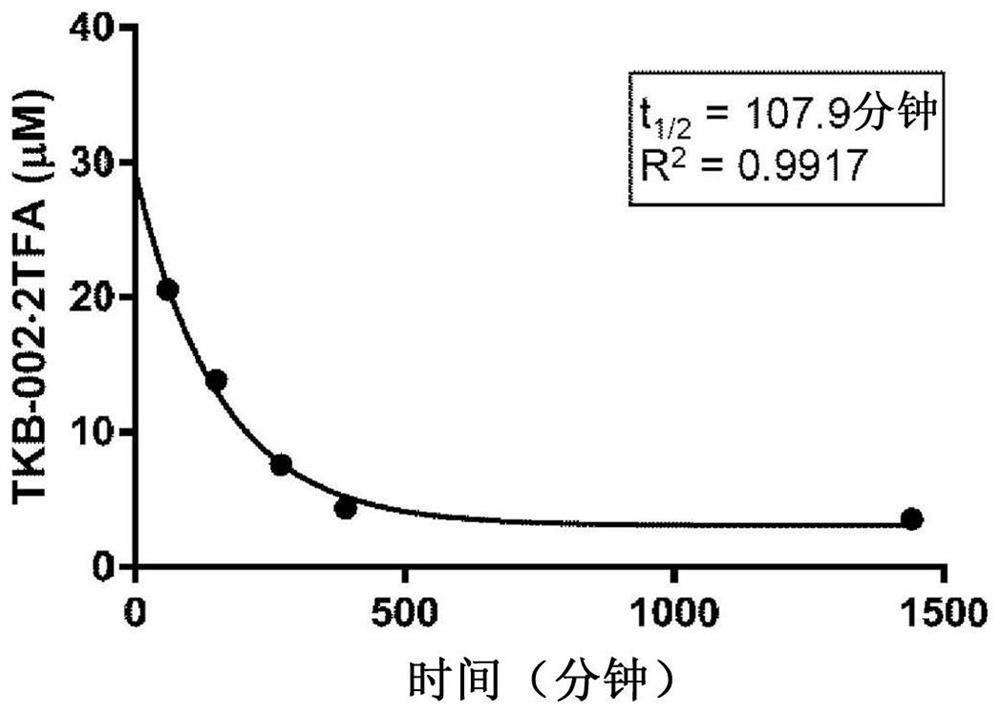
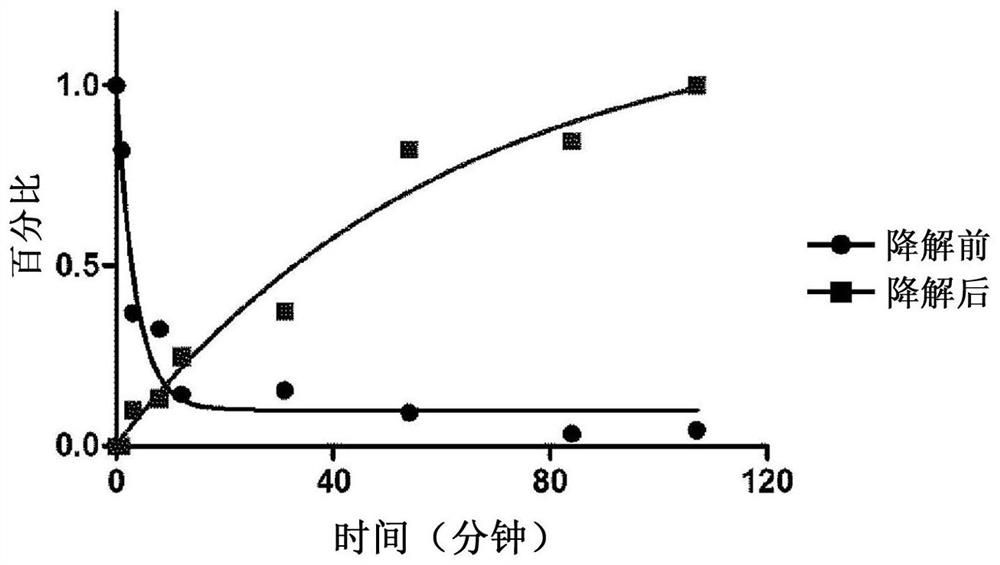
CD4 mimetic compounds with anti-HIV activity
A compound, HIV-1 technology, applied in organic active ingredients, drug combinations, organic chemistry, etc., can solve the problems of high cytotoxicity, low water solubility, and low anti-HIV activity, and achieve low cytotoxicity and high anti-HIV activity. , the effect of prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] [Example 1 Synthesis 1 of the compound of the present invention]
[0099] Compounds of the present invention with PEG chains were synthesized by the following synthetic scheme of steps.
[0100] [Chemical formula 12]
[0101]
[0102] To a solution of compound 1 (13.0 g, 20.0 mmol) in MeOH (67 mL) was added SOCl at 0 °C 2 (1.6 mL, 22 mmol). The reaction mixture was stirred at room temperature for 17 hours, then the mixture was concentrated under reduced pressure to give a crude mixture as a white powder. to CH 2 Cl 2 To the crude mixture in (219 mL) was added piperidine (9.32 g, 109.5 mmol). The reaction mixture was stirred at room temperature for 2 hours and then subjected to silica gel column chromatography (CHCl). 3 / MeOH=10 / 1) to obtain compound 3 (N ω -((2,2,4,6,7-Pentamethyl-2,3-dihydrobenzofuran-5-yl)sulfonyl)-L-arginine methyl ester) as a white powder (7.61 g , the yield is 87%).
[0103] 1 H NMR (500MHz, CDCl 3 )δ1.45(s,6H),1.59-1.66(m,2H),1.79-1....
Embodiment 2
[0114] [Example 2 Synthesis 2 of the compound of the present invention]
[0115] In the same manner as in Example 1, compounds with different PEG chain lengths were synthesized.
[0116] [Chemical formula 16]
[0117]
[0118] To compound 4 (1.06 g, 1.97 mmol) in CHCl at 0 °C 3 (17.9mL) HOBt·H was added to the solution 2 O (301.7 mg, 1.97 mmol), EDCI·H 2 O (0.378 g, 1.97 mmol), m-PEG11-amine (1.00 g, 1.79 mmol), and DIPEA (0.61 mL, 3.58 mmol). The reaction mixture was stirred at room temperature overnight, then saturated NH was added 4 The reaction was quenched with aqueous Cl, and the reaction was quenched with CH 2 Cl 2 extraction, then the organic phase was passed over MgSO 4 Concentrate to dryness. To the crude mixture in THF (19.7 mL) was added IN aqueous LiOH (0.39 mL) at room temperature. The reaction mixture was stirred at room temperature for 30 minutes, then the mixture was filtered, quenched by the addition of 1N aq. 3 extraction. Pass the organic phas...
Embodiment 3
[0123] [Example 3 Synthesis 3 of the compound of the present invention]
[0124] In the same manner as in Example 1, compounds with different PEG chain lengths were synthesized.
[0125] [Chemical formula 18]
[0126]
[0127] To CH of compound 4 (49 mg, 91.9 μmol) at 0 °C 2 Cl 2 (91 μL) was added m-PEG23-amine (100 mg, 91.9 μmol), EDCI·H 2 O(19.4mg, 101μmol), HOBt·H 2 O (13.7 mg, 101 μmmol), and DIPEA (32 μL, 184 μmol). The reaction mixture was stirred at room temperature overnight, then washed with saturated NH 4 The reaction was quenched with aqueous Cl, and the reaction was quenched with CH 2 Cl 2 extraction, then the organic phase was passed over MgSO 4 Concentrate to dryness. To the crude mixture in THF (426 μL) was added IN aqueous LiOH (92.2 μL) at room temperature. The reaction mixture was stirred at room temperature for 30 minutes, then the mixture was filtered and washed with NH 4 Aqueous Cl quenched with CHCl 3 extraction. Pass the organic phase ove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com