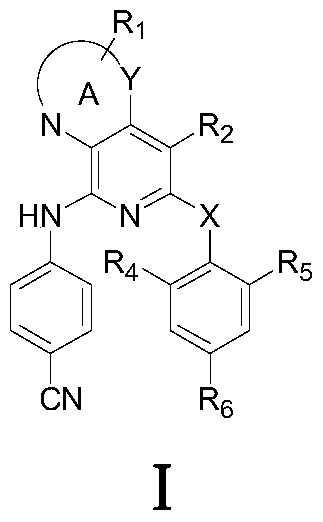
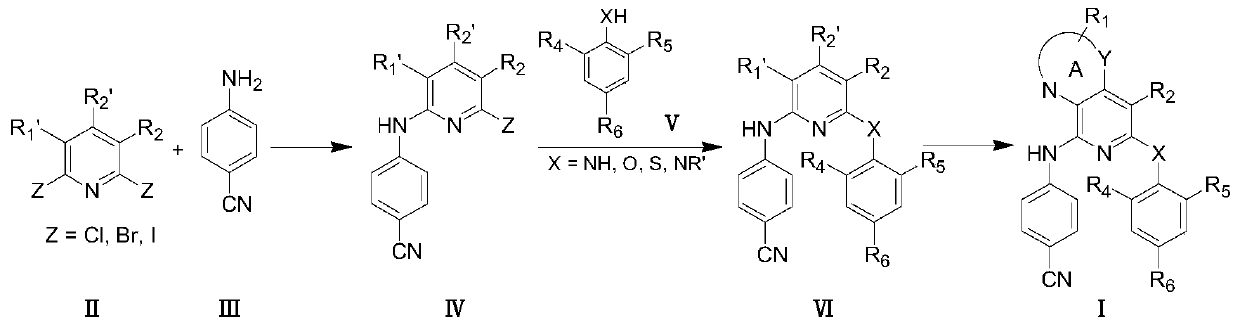
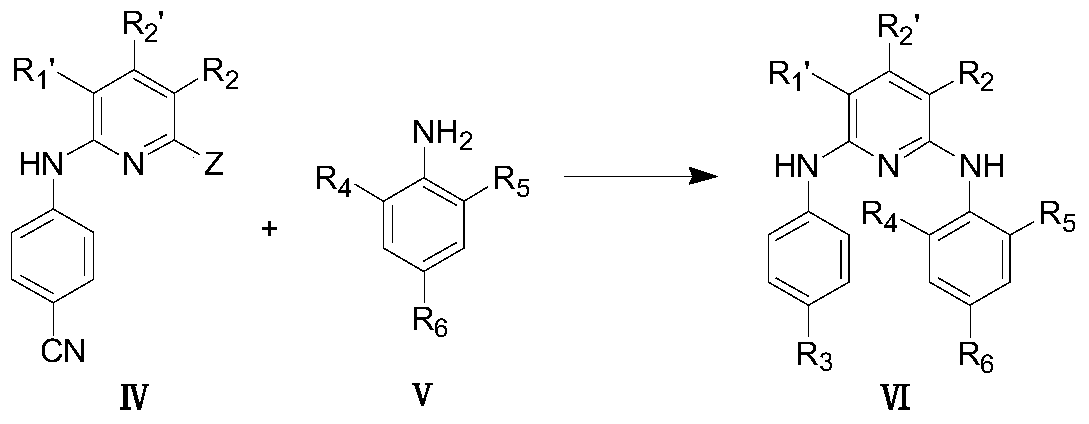
Pyridine condensed ring compound and its preparation method and use
Technology of a compound, pyridine, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0191] Preparation Example 1 2-(4-cyanoanilino)-3-nitro-4-amino-6-chloro-pyridine (IV-1)
[0192]
[0193] 2,6-dichloro-3-nitro-4-aminopyridine (II-1, 416mg, 2.0mmol) and p-cyanoaniline (III-1, 472mg, 4.0mmol), solvent-free reaction, react overnight at 100°C, add Dissolve DMF, introduce into water with pH=3.0, stir for 10 minutes, filter with suction, filter the solid, stir in aqueous sodium bicarbonate solution for 15 minutes, filter with suction, wash with water, dry, separate and purify with medium pressure column (dichloromethane, ethyl acetate is eluent) to obtain 491.3 mg of a yellow solid with a yield of 85%.
preparation Embodiment 2
[0194] Preparation Example 2 2-(4-cyanoanilino)-3-nitro-4-amino-5-bromo-6-chloro-pyridine (IV-2)
[0195]
[0196] Using 4-amino-3-nitro-2,6-dichloro-5-bromopyridine as raw material, the preparation method is the same as IV-1, and the yield is 90%.
preparation Embodiment 3
[0197] Preparation Example 3 2-(4-cyanoanilino)-3-nitro-4-amino-5,6-dichloropyridine (IV-3)
[0198]
[0199] Using 4-amino-3-nitro-2,5,6-trichloropyridine as raw material, the preparation method is the same as IV-1, and the yield is 92.75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com