Patents
Literature
122 results about "Nucleoside Analog Reverse Transcriptase Inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Nucleoside analog reverse-transcriptase inhibitors (NARTIs or NRTIs) compose the first class of antiretroviral drugs developed. In order to be incorporated into the viral DNA, NRTIs must be activated in the cell by the addition of three phosphate groups to their deoxyribose moiety, to form NRTI triphosphates.
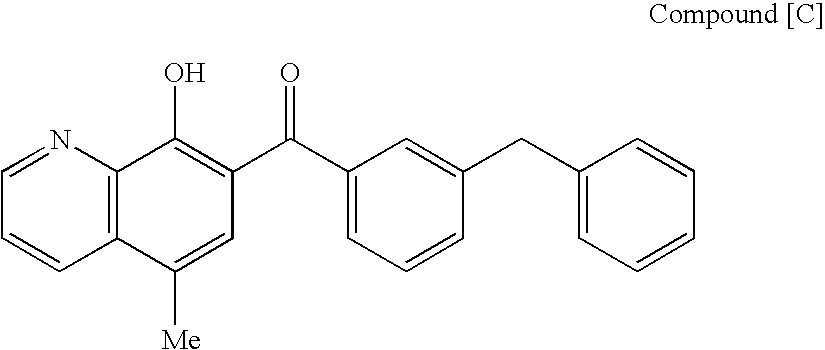
4-oxoquinoline compound and use thereof as pharmaceutical agent
Owner:JAPAN TOBACCO INC
Topical Antiviral Formulations
InactiveUS20080035155A1Reduce riskPrevent and reduce riskBiocideOrganic active ingredientsTopical antiviralReverse transcriptase
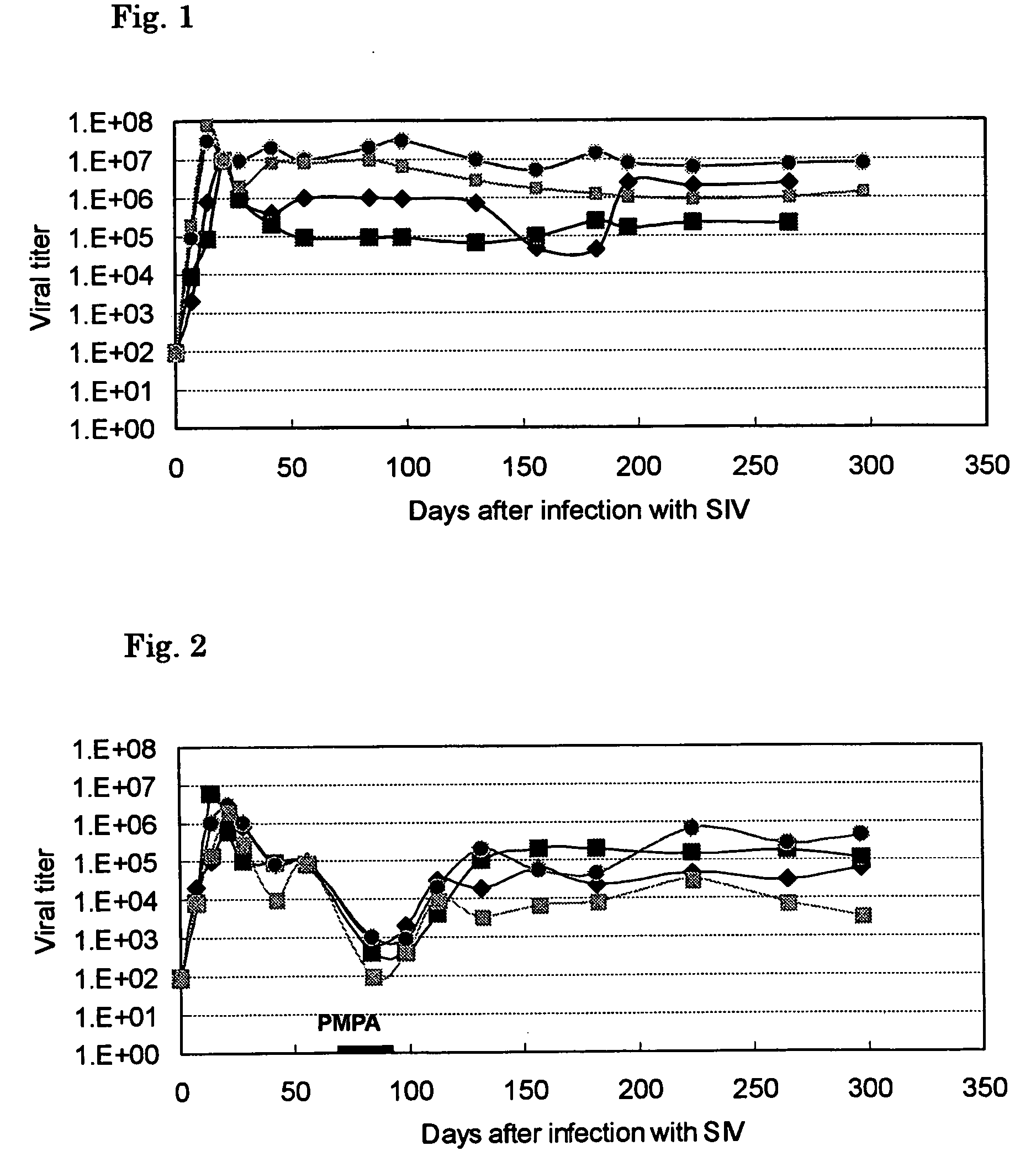
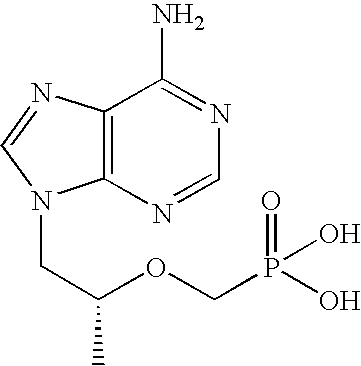
The present invention relates to formulations of nucleotide reverse transcriptase inhibitors (N(t)RTIs), preferably [2-(6-Amino-purin-9-yl)-1-methyl-ethoxymethyl]-phosphonic acid (tenofovir, PMPA), or a physiologically functional derivative thereof, suitable for topical application and their use in the prevention of HIV infections.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL +1
HIV reverse transcriptase inhibitors
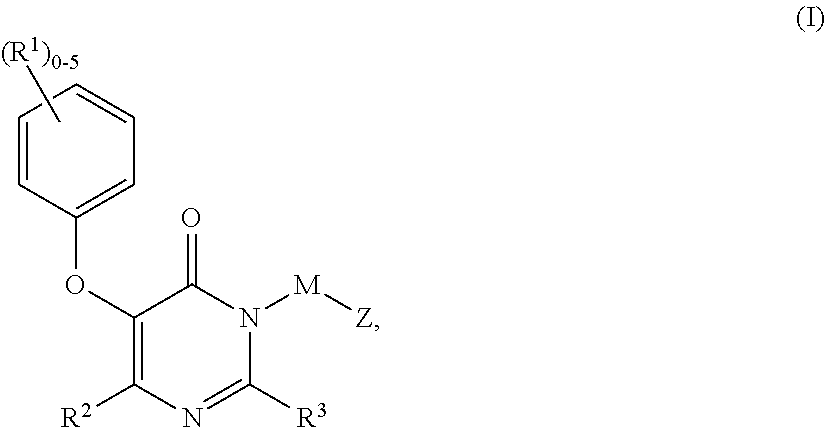
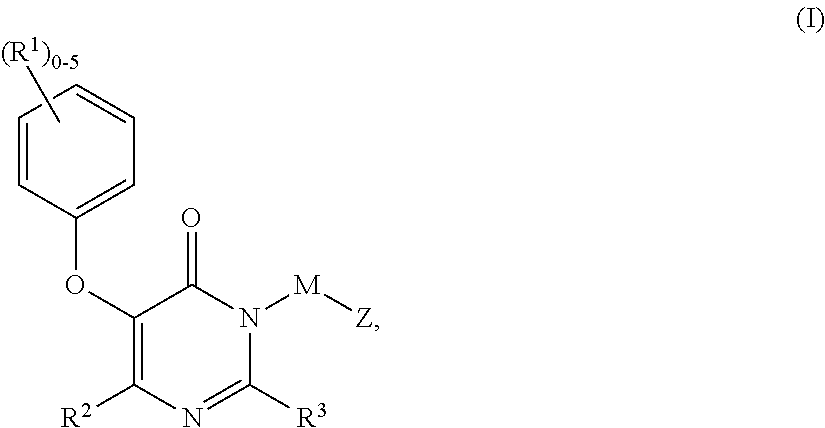
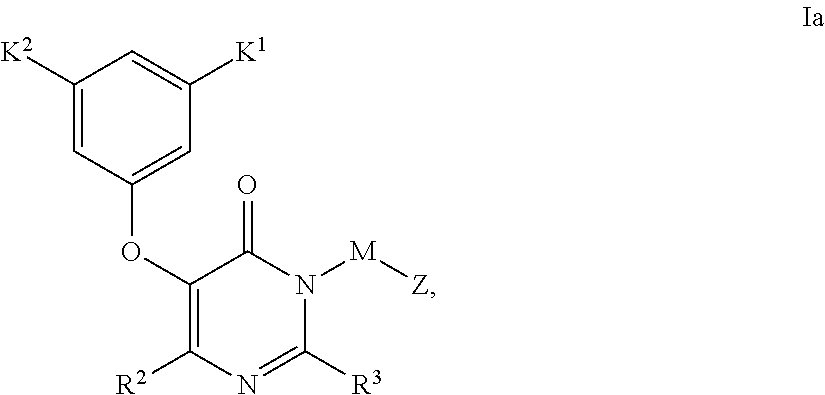
Compounds having the structure: are HIV reverse transcriptase inhibitors, wherein A, X, Y, Z, R1 and R2 are defined herein. The compounds and their pharmaceutically acceptable salts are useful in the inhibition of HIV reverse transcriptase, the prophylaxis and treatment of infection by HIV and in the prophylaxis, delay in the onset, and treatment of AIDS. The compounds and their salts can be employed as ingredients in pharmaceutical compositions, optionally in combination with other antivirals, immunomodulators, antibiotics or vaccines.
Owner:MERCK SHARP & DOHME CORP
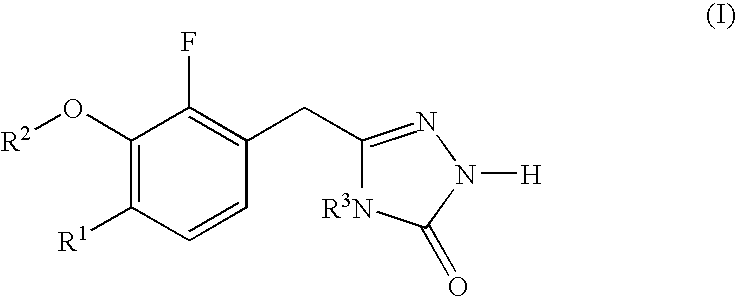
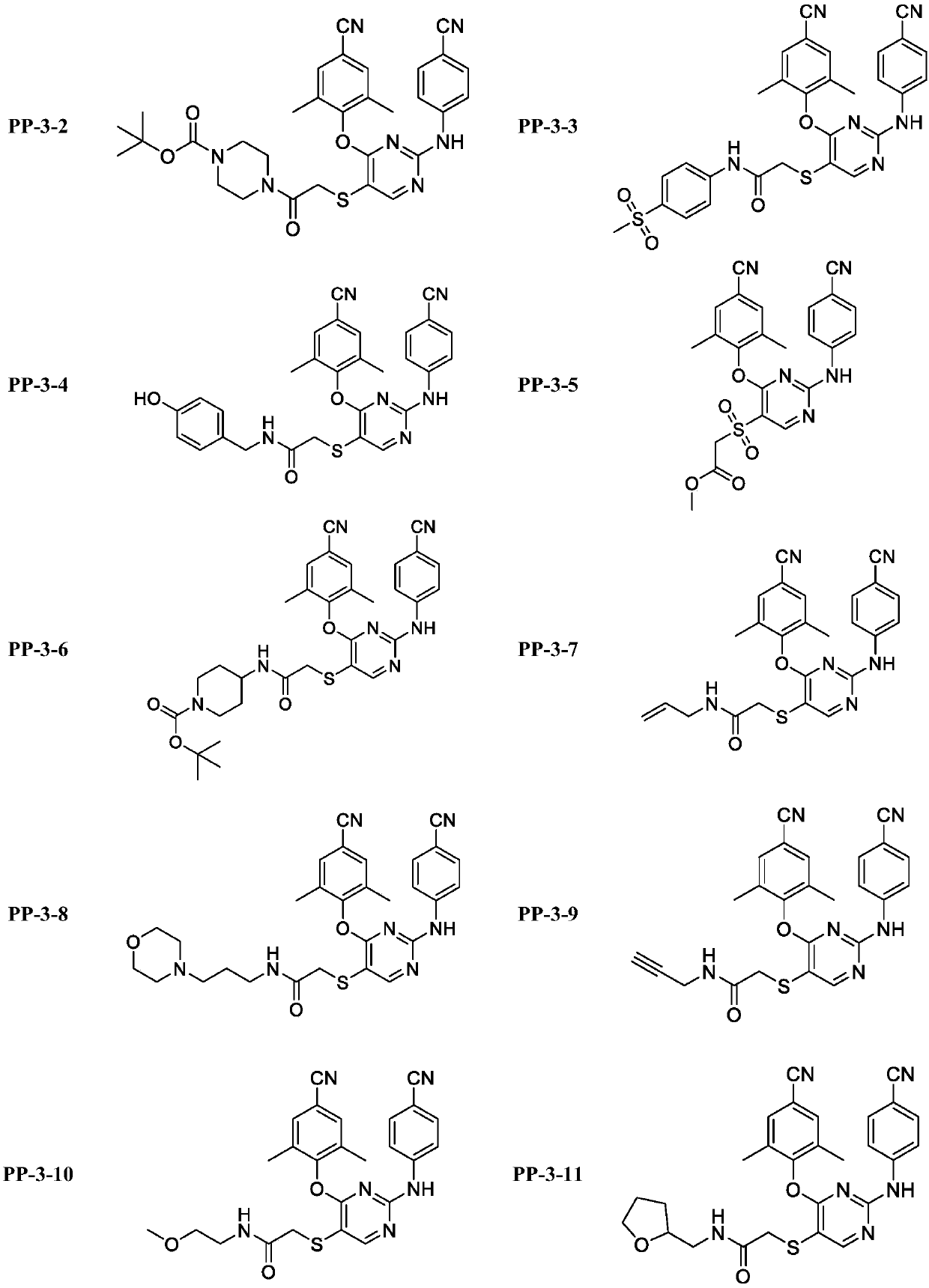
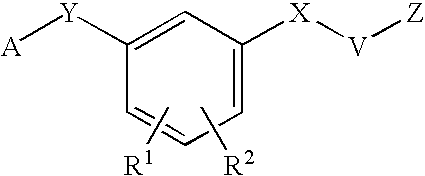
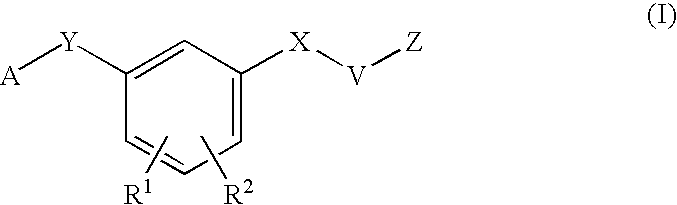
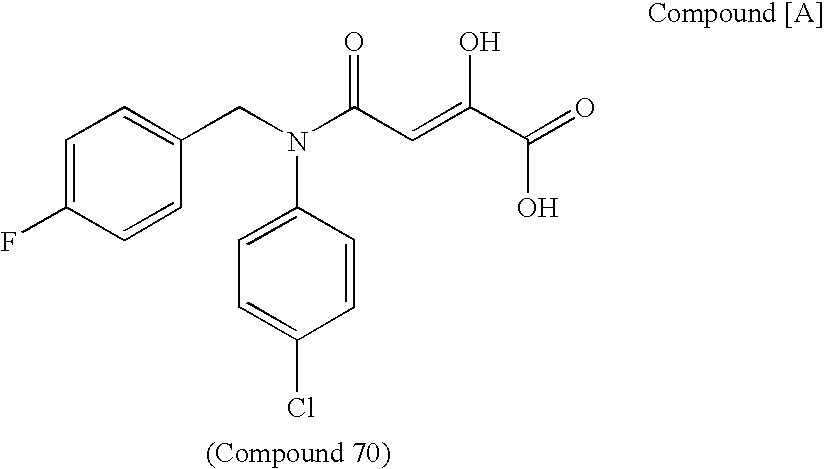
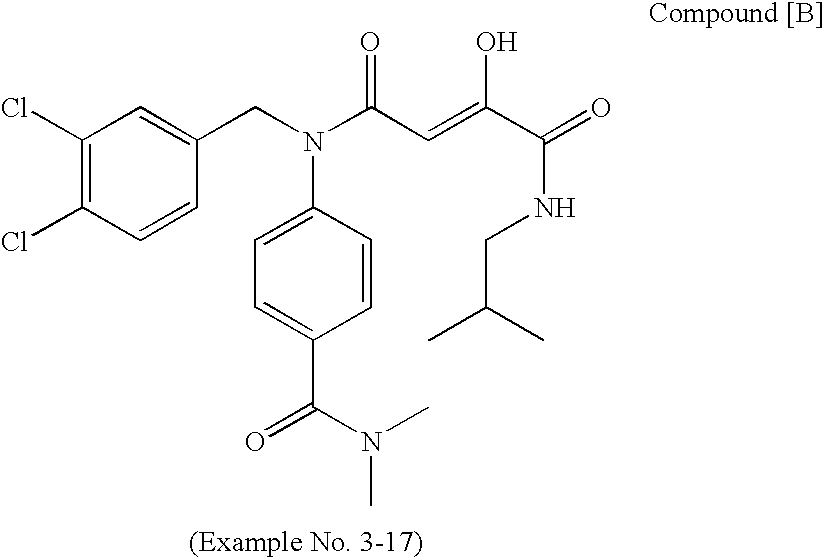
N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-p-amino benozioc acids as HIV reverse transcriptase inhibitors
Owner:ARDEA BIOSCIENCES INC
Nitrogen-containing fused ring compound and use thereof as HIV integrase inhibitor
ActiveUS20060052361A1Effective anti-HIV agentStrong inhibitory activityBiocideOrganic chemistryAnti-HIV AgentSide effect
Owner:JAPAN TOBACCO INC
Combination therapy
ActiveUS20050288326A1Low effective doseLow cytotoxicityBiocideAntiviralsSide effectReverse transcriptase
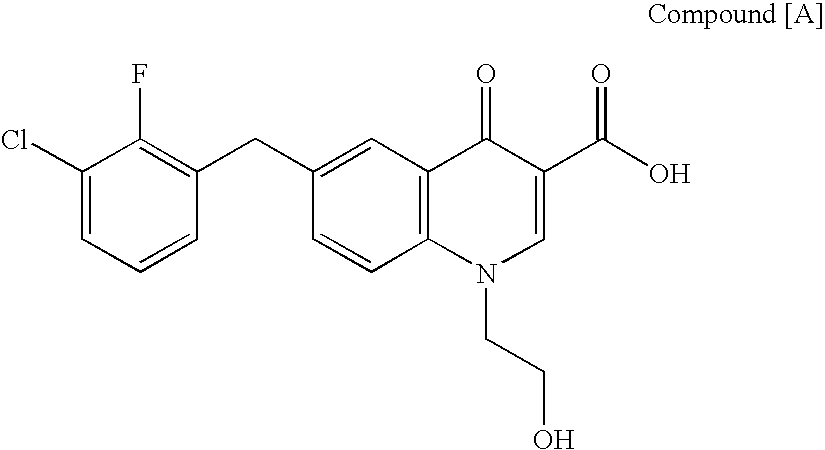
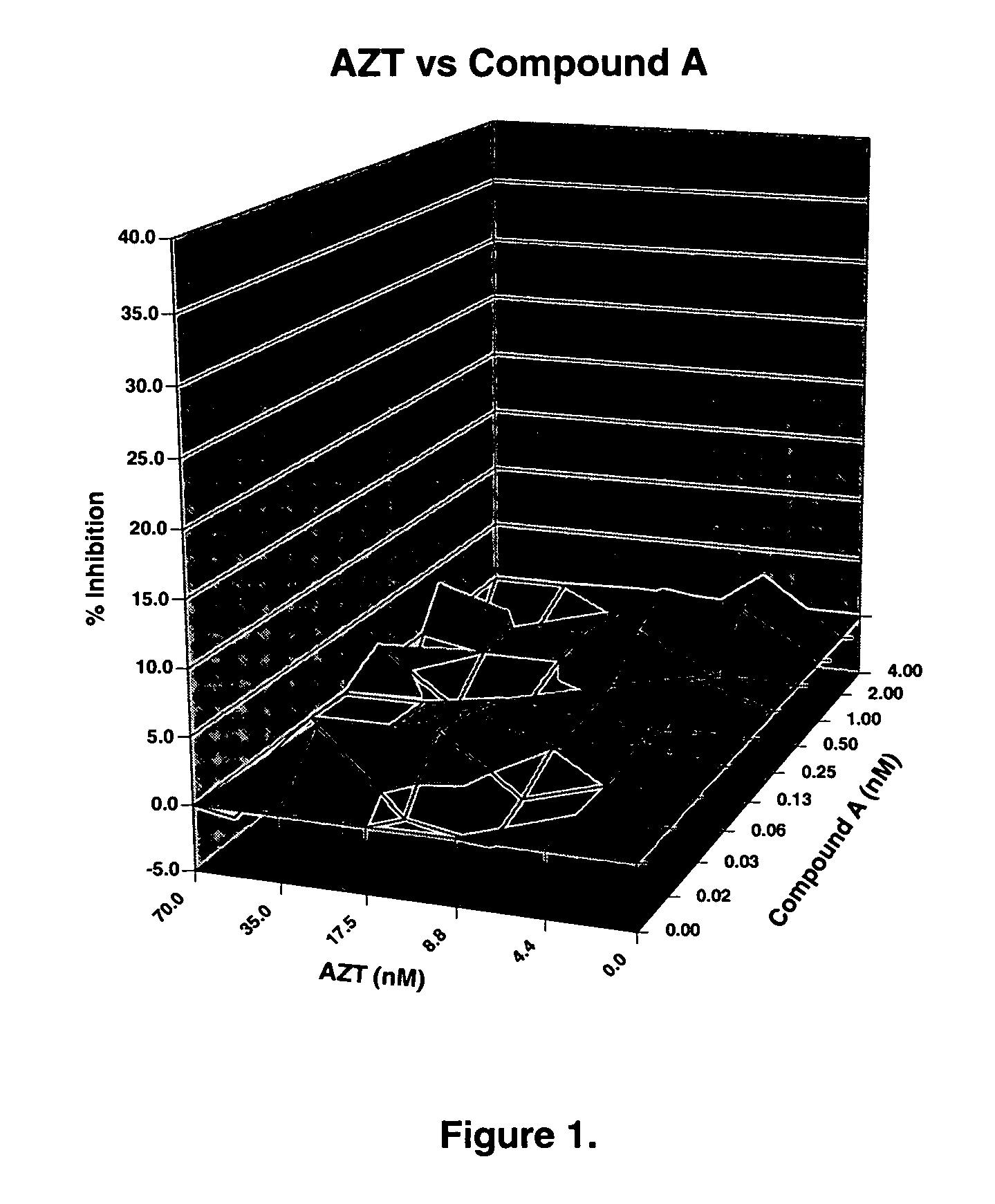
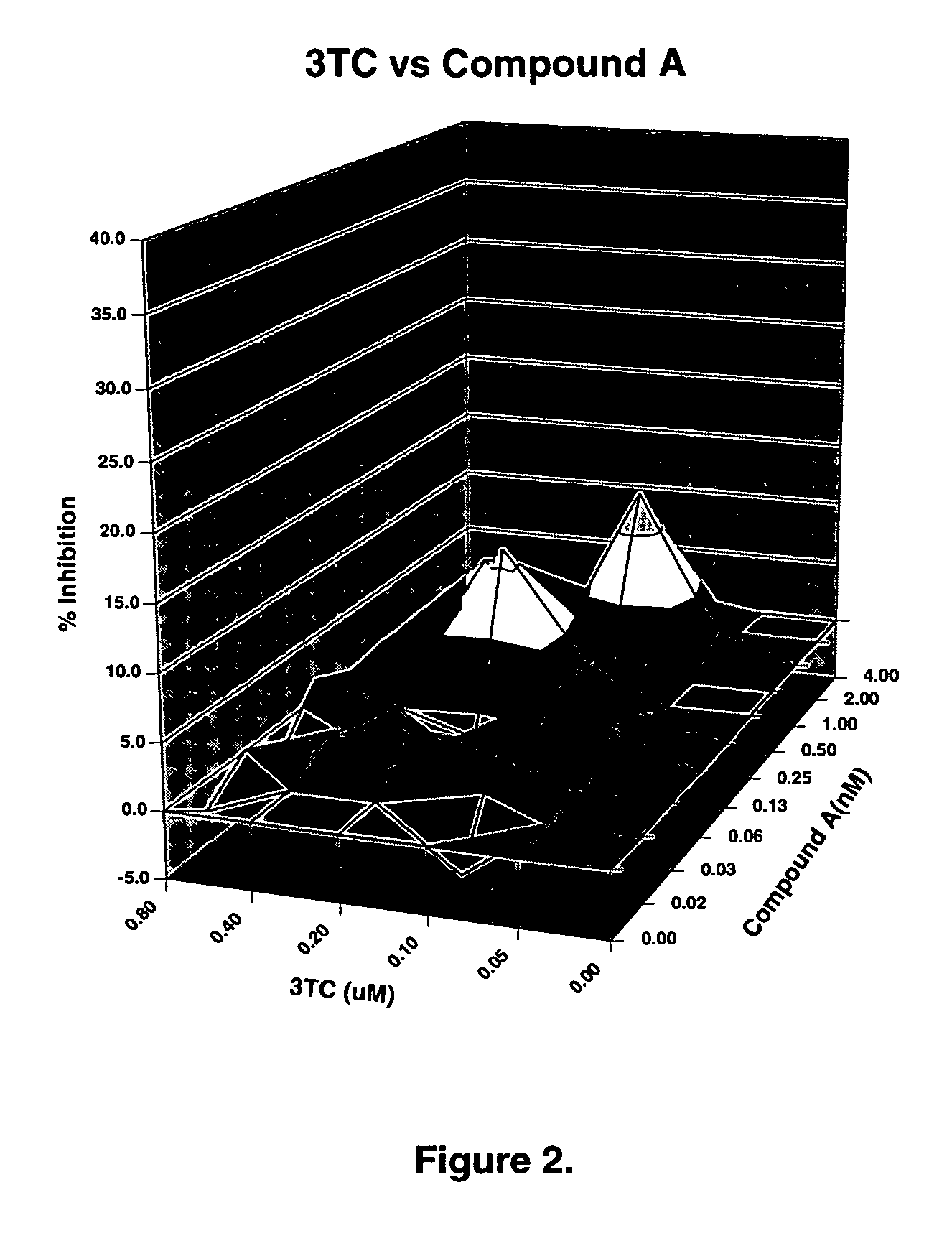
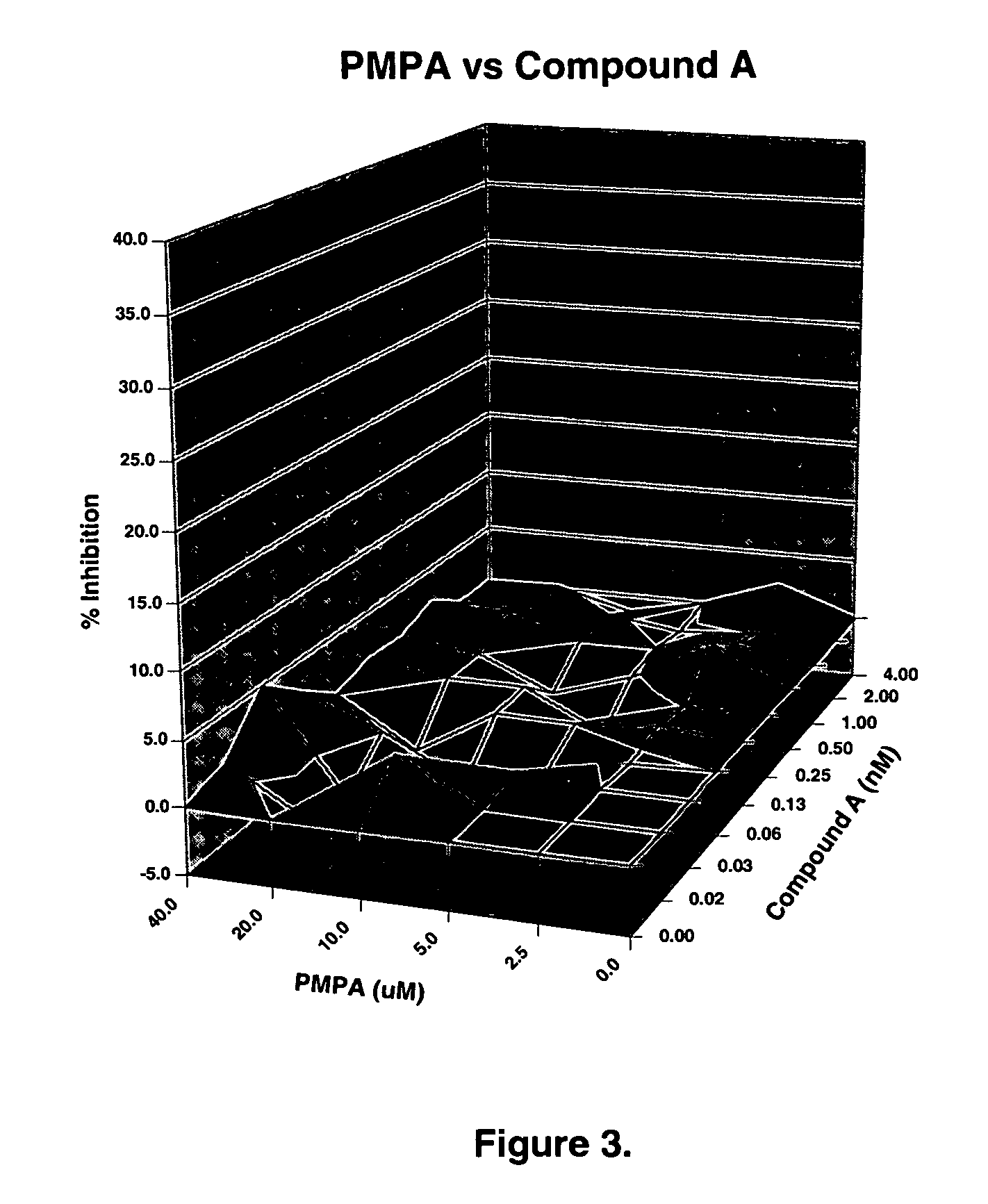
The present invention relates to a combination therapy for treating an HIV infection or inhibiting integrase comprising (S)-6-(3-Chloro-2-fluorobenzyl)-1-(1-hydroxymethyl-2-methylpropyl)-7-methoxy-4-oxo-1,4-dihydroquinoline-3-carboxylic acid (“Compound A”) or a pharmaceutically acceptable solvate or salt thereof in combination with at least one other anti-HIV agent. In some embodiments of the present invention, the other anti-HIV agents are chosen from reverse transcriptase inhibitors and protease inhibitors. In certain embodiments of the present invention, the other anti-HIV agents are chosen from AZT, 3TC, PMPA, efavirenz, indinavir, nelfinavir, a combination of AZT / 3TC, and a combination of PMPA / 3TC. Since Compound A has a high inhibitory activity specific for integrases, when used in combinations with other anti-HIV agents it can provide a combination therapy with fewer side effects for humans.
Owner:JAPAN TOBACCO INC
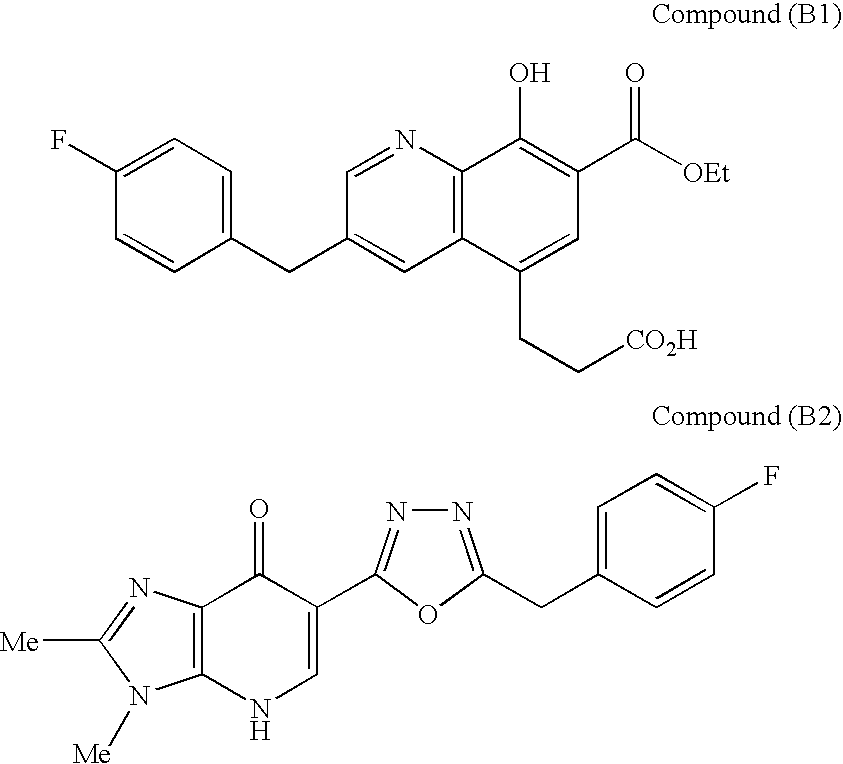
N[S(4-aryl-triazol-3-yl)alpha-mercaptoacetyl]-p-amino benzoic acids as HIV reverse transcriptase inhibitors
A series of S-triazolyl α-mercaptoacetanilides having N-(α-mercaptoacetyl) p amino benzoic acid derivatives. are provided, where Q is CO2H, or a salt or ester thereof, or a C(O)N-linked amino acid. The compounds inhibit several variants of the reverse transcriptase of HIV, and are useful in the treatment of HIV infections.
Owner:ARDEA BIOSCI
Non-nucleoside reverse transcriptase inhibitors
Heteroaromatic compounds of Formula I:are HIV reverse transcriptase inhibitors, wherein R1, R2, R3, R4 and R5 are defined herein. The compounds of Formula I and their pharmaceutically acceptable salts are useful in the inhibition of HIV reverse transcriptase, the prophylaxis and treatment of infection by HIV and in the prophylaxis, delay in the onset or progression, and treatment of AIDS. The compounds and their salts can be employed as ingredients in pharmaceutical compositions, optionally in combination with other antivirals, immunomodulators, antibiotics or vaccines.
Owner:MERCK CANADA
Combination therapy comprising the use of protein kinase C modulators and Histone Deacetylase inhibitors for treating HIV-1 latency
InactiveUS20100166806A1Adverse propertyPrevent HIV-1-induced cytotoxicityBiocideOrganic chemistryReverse transcriptaseHydroxamic acid
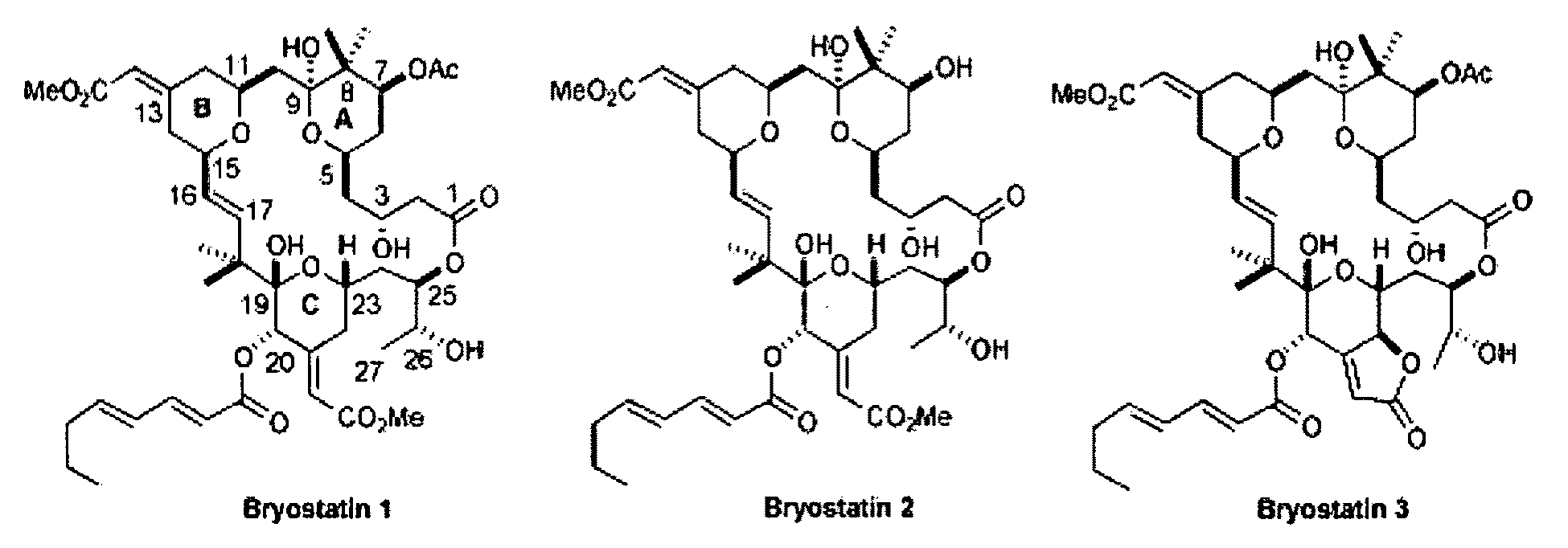
The invention relates to a combination of treatments, more particularly a combination treatment for HIV-1 infection. The present invention is directed to the use of bryostatin-1 and their natural and synthetic derivatives for AIDS therapy, in particular to the use of bryostatins in combination with other active drugs such as Histone Deacetylases (HDACs) inhibitors and anti-retrovirals, for the treatment of HIV-1 latency. According to the present invention, we provide a combination therapy for the treatment of HIV-1 latency which employs bryostatin-1 (and analogues) and one of the following HDAC inhibitors; valproic acid, butyrate derivatives, hydroxamic acids and benzamides. While HDACi can be used in continuous dosing protocol, bryostatins can be used following a cyclical dosing protocol. Bryostatins can be formulated in pharmaceutical acceptable carriers including nanoparticles, phospholipids nanosomes and / or biodegradable polymer nanospheres. This combination therapy needs to be used in patients treated with antiretroviral therapy (HIV-1 protease inhibitors, HIV-1 reverse transcriptase inhibitors, HIV-1 integrase inhibitors, CCR5 co-receptor inhibitors and fusion inhibitors).
Owner:APHIOS
Combination therapy
ActiveUS8633219B2Low effective doseEffective treatmentBiocideOrganic chemistrySide effectCombined Modality Therapy
Owner:JAPAN TOBACCO INC
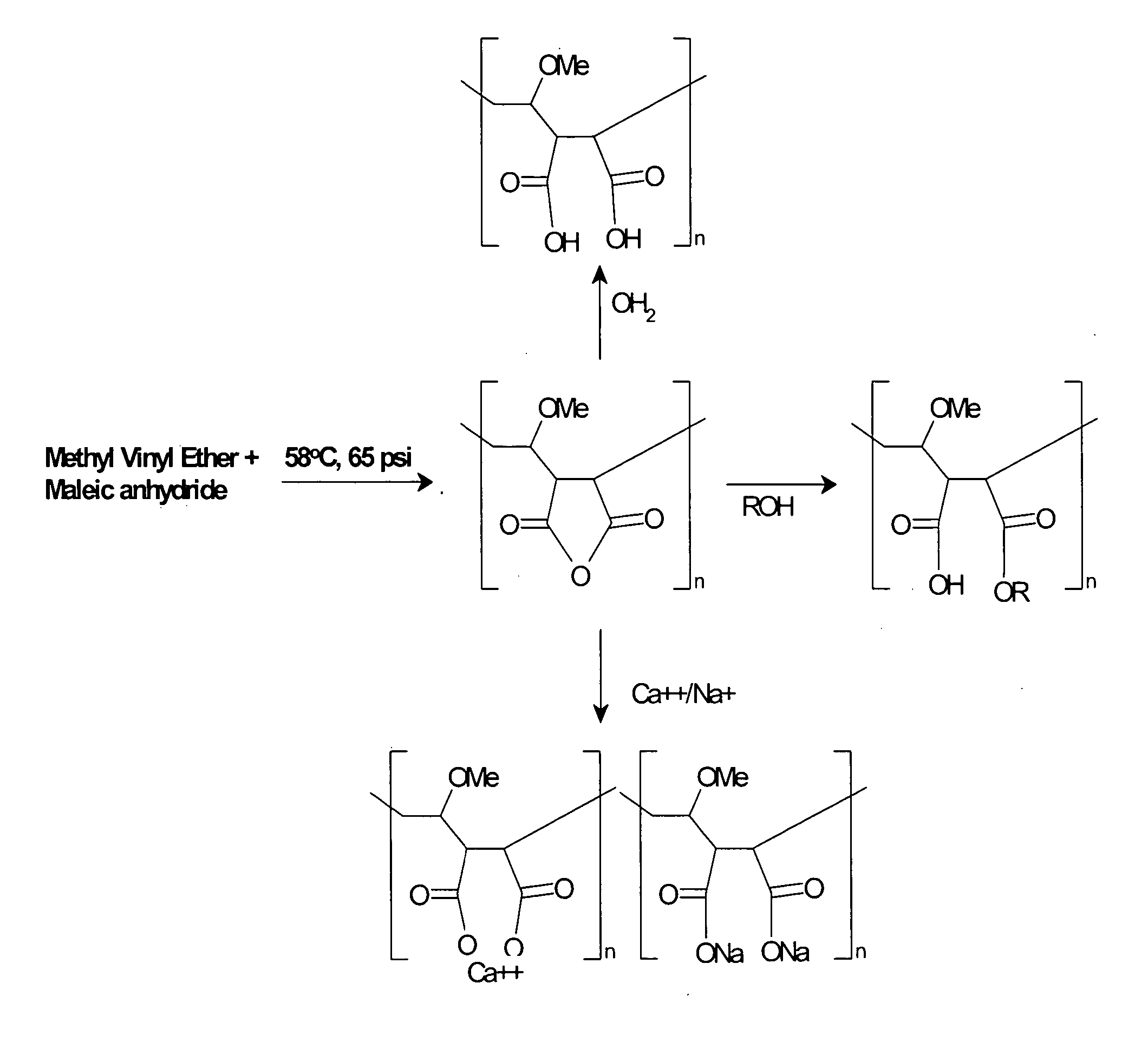
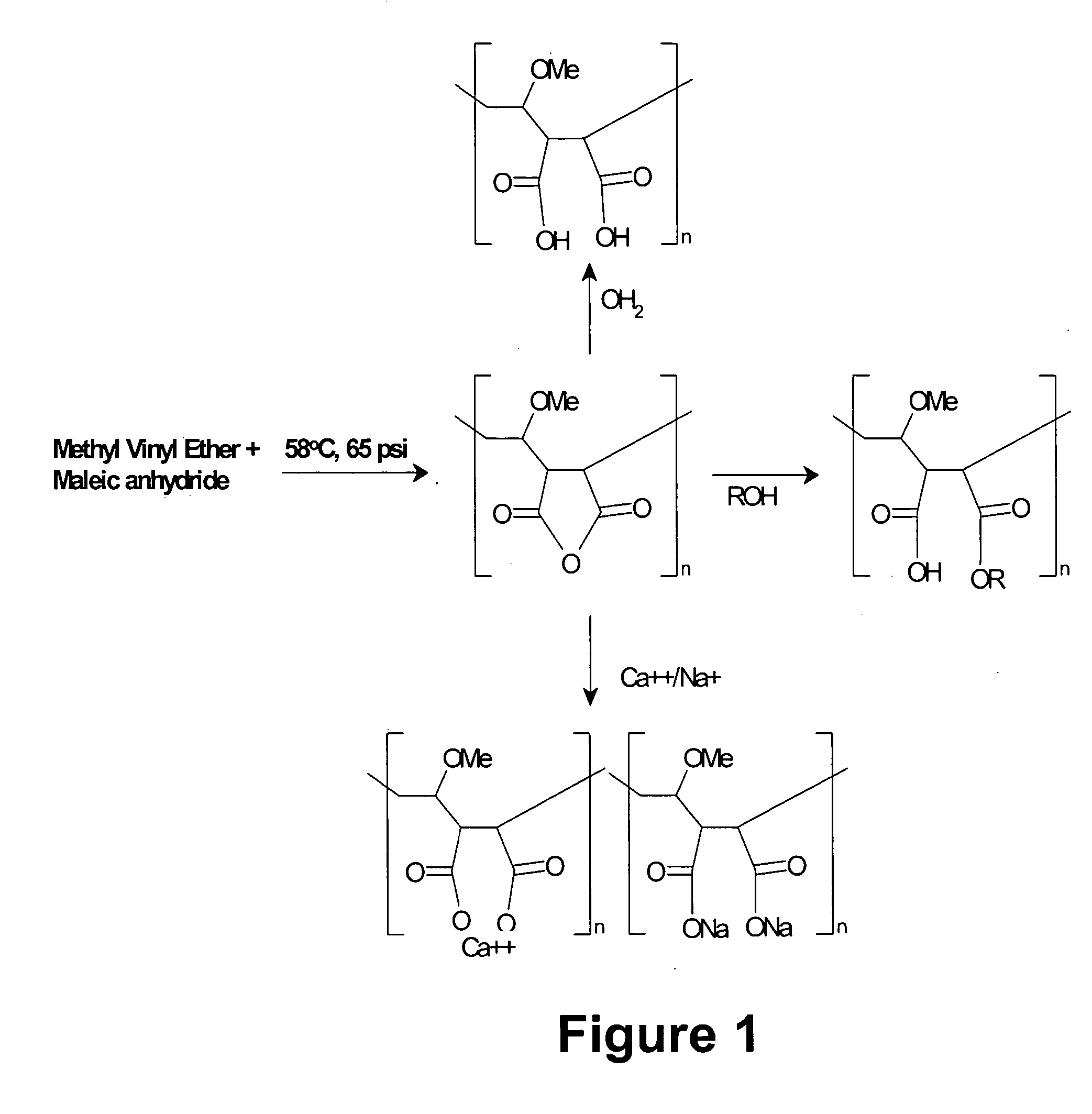
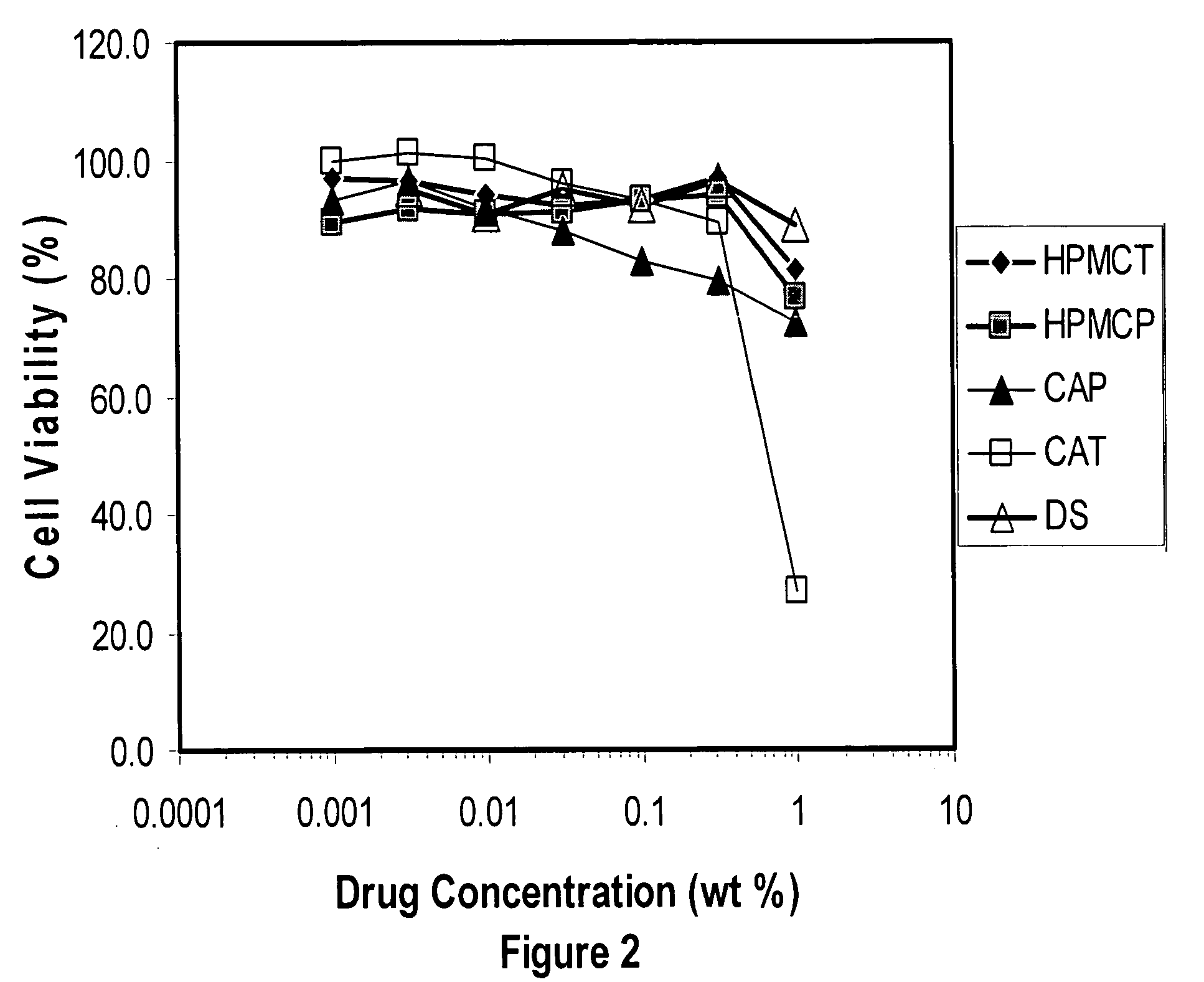
Methods, compositions, formulations, and uses of cellulose and acrylic-based polymers
InactiveUS20050244365A1Easy to chargeLow pKaAntibacterial agentsCosmetic preparationsDisinfectantReverse transcriptase
Compositions, formulations, and methods for the treatment or prevention, or decreasing the frequency of transmission of a virus (such as human immunodeficiency virus type 1 (HIV-1), Herpes Simplex virus type 1 (HSV1), or Herpes Simplex Virus Type 2 (HSV2), or other virus), or a bacterial infection (such as Trichomonas vaginalis, Neisseris gonorrhoeae Haemopholus ducreyl, or Chlamydia trachomatis, or other bacterial species), or a fungal infection, using an anionic cellulose- or acrylic-based oligomer, polymer, or copolymer. The present invention also includes administering a therapeutically effective amount of said oligomer, polymer, or copolymer, or a pharmaceutically acceptable salt thereof, or with a pharmaceutically acceptable carrier or diluent, thereof. The invention relies on the unique biochemical substitution of the cellulose or acrylic backbone such that the resultant molecule can remain molecularly dispersed in solution (or gel or other formulation) and mostly dissociated over a wide range of physiological microenvironments, such as the low pH found within the vaginal lumen, preferably from a pH of 14 to below 3.5. These specific substitutions also impart on the resultant molecule potent antiviral, anti-bacterial, and anti-fungal properties. In addition, these compositions can be used as general disinfectants for human use such as in contact lens solutions, mouthwashes, toothpastes, suppositories, or as more generalized disinfectants found in soaps, household cleaning products, paints, water treatments modalities, or can be incorporated into cosmetic, and can be used as vehicles for drug delivery, an adjuvant in a therapeutic formulation, or as a preservative. These compounds can be delivered in a liquid or solid dosage form and can be incorporated into barrier devices such as condoms, diaphragms, or cervical caps, to help prevent the transmission of STDs. The compounds of this invention can also be used in combination therapies with other classes of antiviral, antibacterial, or antifungal agent having similar or differing mechanisms of action including, but not limited to, anionic or cationic polymers, copolymers, or oligomers, surfactants, protease inhibitors, DNA or RNA polymerase inhibitors (including reverse transcriptase inhibitors), fusion inhibitors, cell wall biosynthesis inhibitors, integrase inhibitors, or virus or bacterial attachment inhibitors.
Owner:NOVAFLUX INC +1
Composition for oral cavity and skin
InactiveUS20100239690A1Inhibit wearEasy to cleanAntibacterial agentsBiocideAnticarcinogenSide effect
A composition for oral cavity and skin which contains an antibacterial agent and does not have adverse side effects due to steroids, is provided.The composition contains 0.01 to 4.5% by mass of at least one selected from the group consisting of an antibacterial agent, an antiviral agent, an anti-HIV agent, a non-nucleic acid-based reverse transcriptase inhibitor, an anticancer agent for external use and a disinfectant; 0.01 to 4.5% by mass of a non-steroidal anti-inflammatory agent; 0.001 to 4.5% by mass of a steroidal anti-inflammatory agent; and 0.001 to 10% by mass of a highly water-absorbent polymer or a cellulose derivative.
Owner:NODA SATOSHI
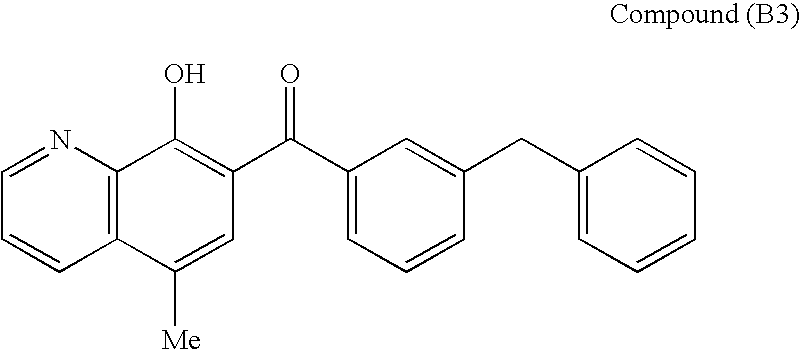
N[S(4-ARYL-TRIAZOL-3-YL)alpha-MERCAPTOACETYL] -P-AMINO BENZOIC ACIDS AS HIV REVERSE TRANSCRIPTASE INHIBITORS
A series of S-triazolyl α-mercaptoacetanilides having N-(α-mercaptoacetyl) p amino benzoic acid derivatives.are provided, where Q is CO2H, or a salt or ester thereof, or a C(O) N-linked amino acid. The compounds inhibit several variants of the reverse transcriptase of HIV, and are useful in the treatment of HIV infections.
Owner:ARDEA BIOSCIENCES INC
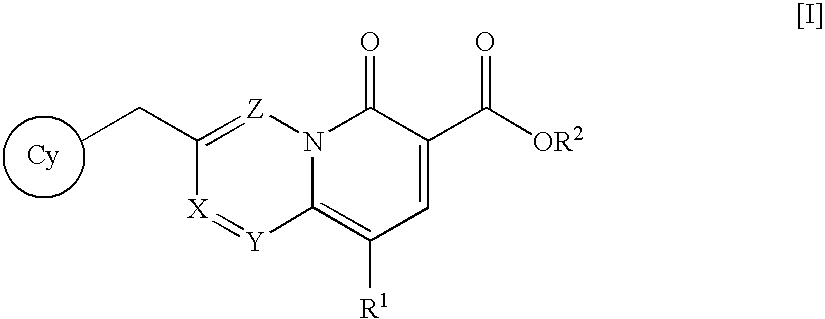
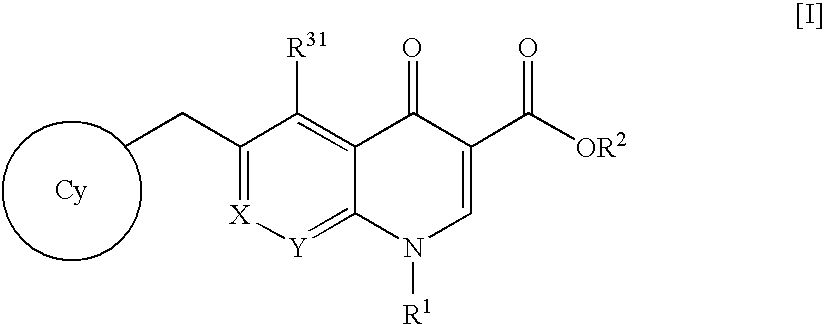
Quinolizinone compound and use thereof as HIV integrase inhibitor
InactiveUS20060084665A1Less side effectsStrong inhibitory activityBiocideOrganic chemistrySide effectReverse transcriptase
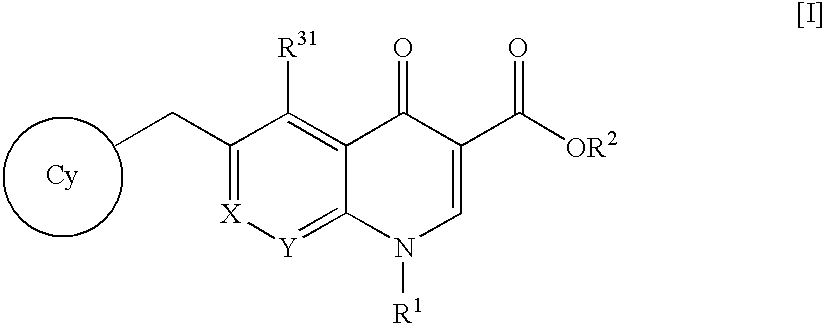
A pharmaceutical agent having an anti-HIV action, particularly, a pharmaceutical agent having an integrase inhibitory action, is provided. The present invention relates to a quinolizinone compound represented by the following formula [I]wherein each symbol is as defined in the specification, a pharmaceutically acceptable salt thereof, and an anti-HIV agent containing same as an active ingredient. The compound of the present invention has an HIV integrase inhibitory action and is useful as an anti-HIV agent for the prophylaxis or therapy of AIDS. Moreover, by a combined use with other anti-HIV agents such as protease inhibitors, reverse transcriptase inhibitors and the like, the compounds can become a more effective anti-HIV agent. Since the compound has a high inhibitory activity specific for integrases, the compound can provide a safe pharmaceutical agent for human with a fewer side effects.
Owner:JAPAN TOBACCO INC
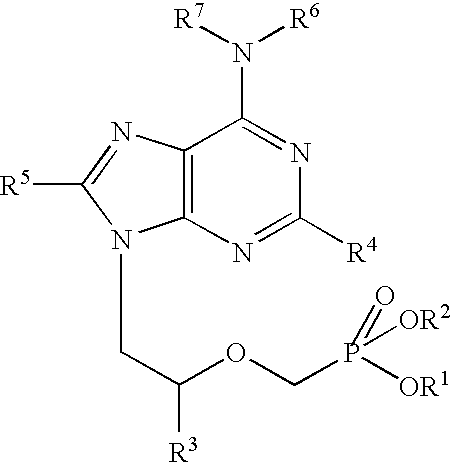
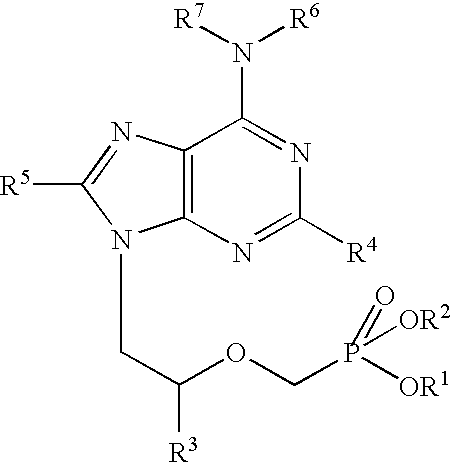
Heterocyclic reverse transcriptase inhibitors
Owner:ROCHE PALO ALTO LLC
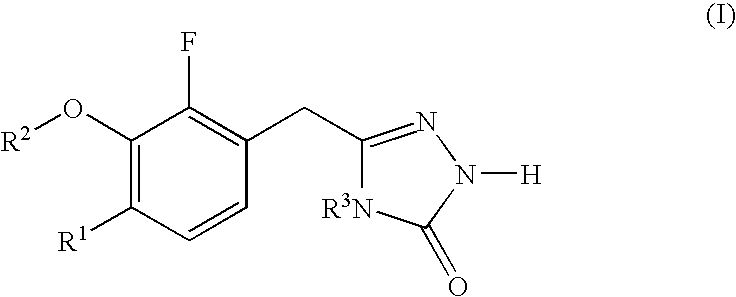
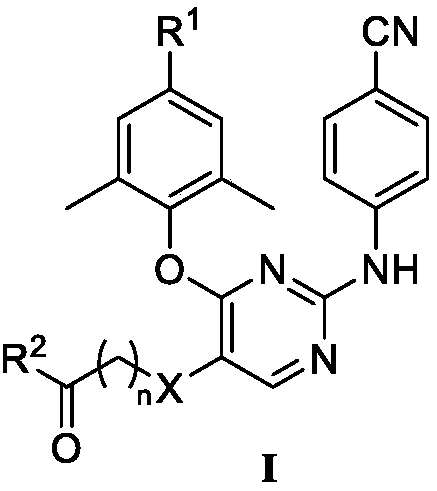
Diarylpyrimidines HIV-1 reverse transcriptase inhibitor as well as preparation method and application thereof
ActiveCN107778255AOrganic active ingredientsOrganic chemistryImmunodeficiency virusNucleoside Analog Reverse Transcriptase Inhibitor
The invention relates to a diarylpyrimidines HIV-1 reverse transcriptase inhibitor as well as a preparation method and application thereof. The diarylpyrimidines HIV-1 reverse transcriptase inhibitorhas a structure shown as a formula I. The invention further relates to a pharmaceutical composition containing the compound with the structure formula I. The invention further provides application ofthe compound and the composition containing one or more compounds to preparation of medicines for treating and preventing human immunodeficiency virus (HIV).
Owner:SHANDONG UNIV
Non-nucleoside reverse transcriptase inhibitors
ActiveUS20140100231A1BiocideOrganic chemistryNucleoside Reverse Transcriptase InhibitorImmunomodulating Agent
Compounds of Formula I:are HIV reverse transcriptase inhibitors, wherein R1, R2, RE, L, M and Z are defined herein. The compounds of Formula I and their pharmaceutically acceptable salts are useful in the inhibition of HIV reverse transcriptase, the prophylaxis and treatment of infection by HIV and in the prophylaxis, delay in the onset or progression, and treatment of AIDS. The compounds and their salts can be employed as ingredients in pharmaceutical compositions, optionally in combination with other antivirals, immunomodulators, antibiotics or vaccines.
Owner:MERCK SHARP & DOHME LLC
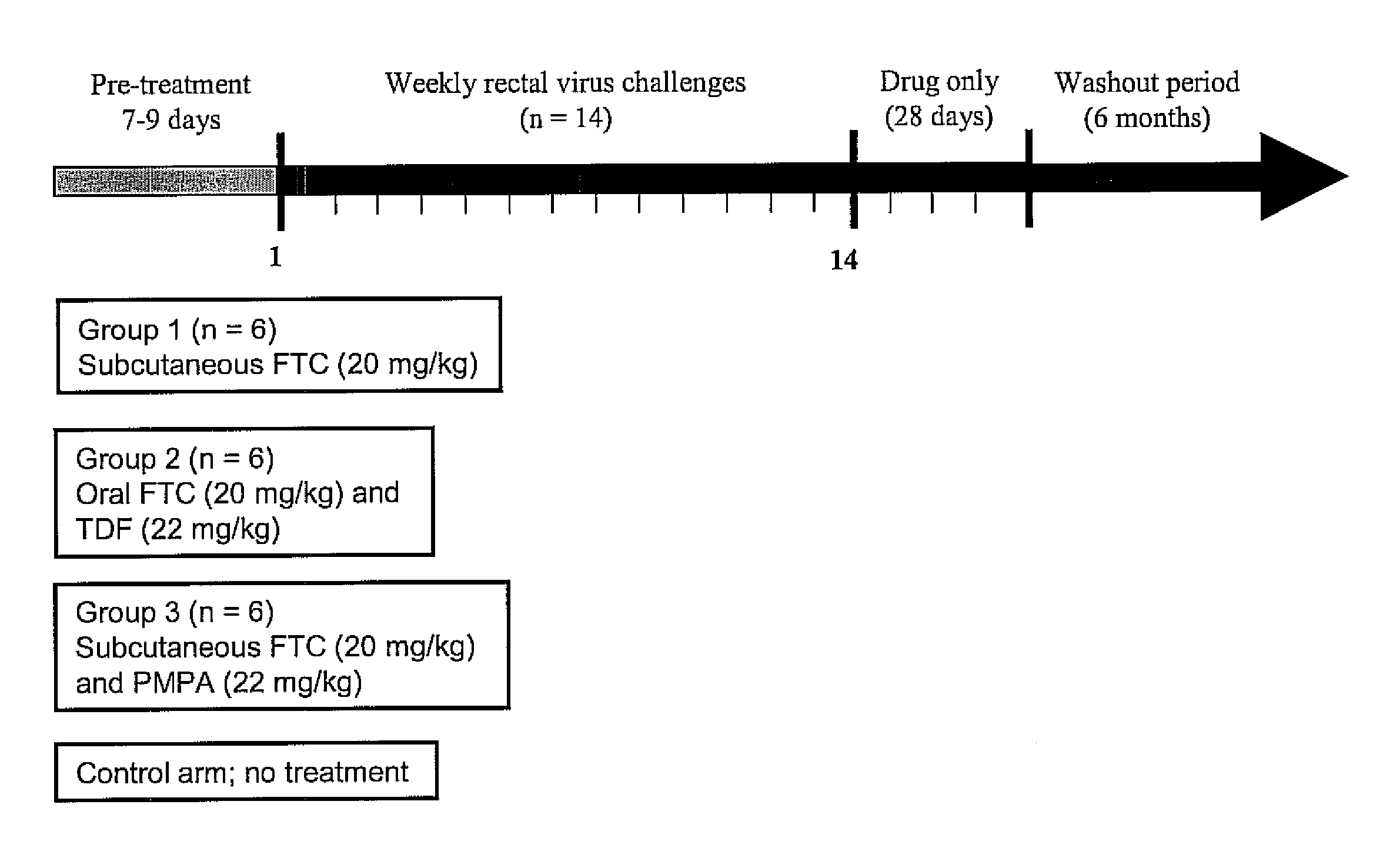
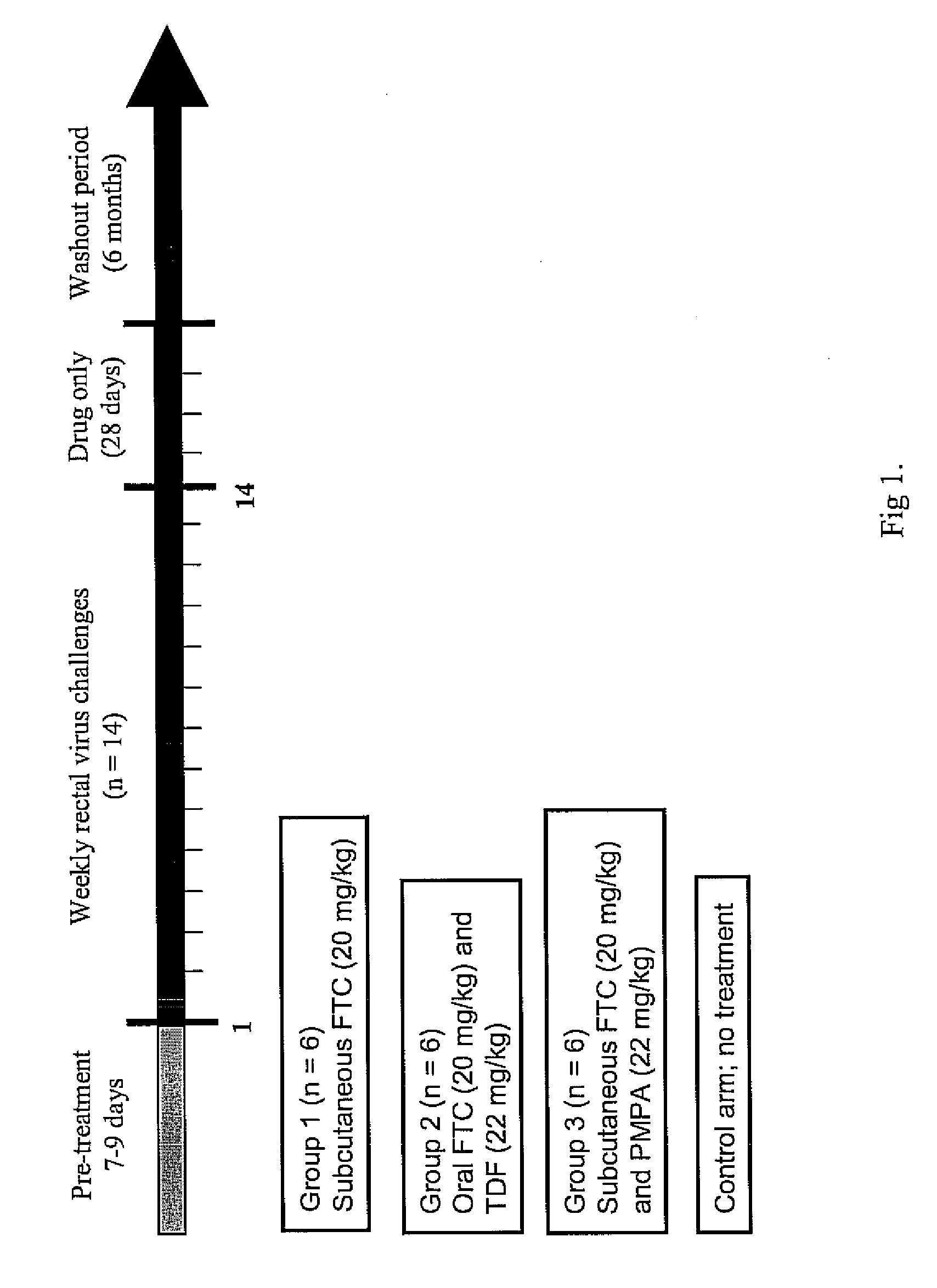
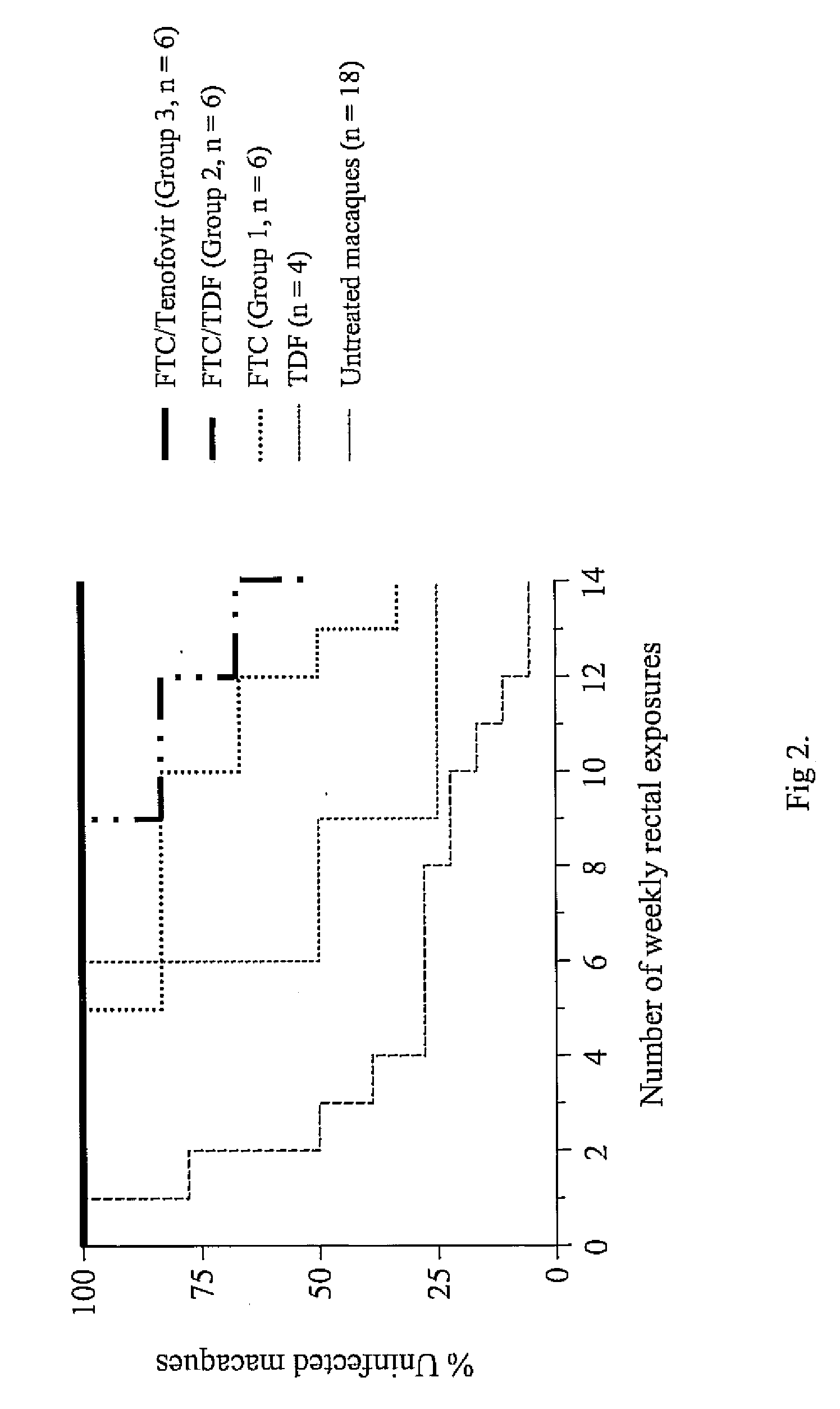
Inhibition of HIV infection fhrough chemoprophyalxis
ActiveUS20070265227A1BiocidePharmaceutical delivery mechanismNucleoside Reverse Transcriptase InhibitorRetroviral infection
A process is provided for protecting a primate host from a self-replicating infection by an immunodeficiency retrovirus. Protection is achieved by administering to the primate host a combination of a pharmaceutically effective amount of a nucleoside reverse transcriptase inhibitor and a pharmaceutically effective amount of a nucleotide reverse transcriptase inhibitor prior to exposure to the immunodeficiency retrovirus. The administration is effective if provided in a single dose within 24 hours of the exposure. A regime of regular daily doses is also effective in providing protection against an immunodeficiency retrovirus becoming self-replicating after infecting a primate host A process for controlling retrovirus transmission within a population includes the administration to a subpopulation at high risk for contracting an immunodeficiency retroviral infection the detailed combination prior to sexual exposure to a source of immunodeficiency retrovirus so as to preclude the immunodeficiency retrovirus from becoming self-replicating in a member of the subpopulation.
Owner:UNITED STATES OF AMERICA
4-Oxoquinoline compound and use thereof as HIV integrase inhibitor
ActiveUS20060217413A1Promote absorptionIncreased riskBiocideOrganic chemistrySide effectReverse transcriptase
Owner:JAPAN TOBACCO INC
Novel HIV integrase inhibitors and HIV therapy based on drug combinations including integrase inhibitors
InactiveUS20050049242A1Strong synergyBiocideAnimal repellantsCombination drug therapyResistant virus
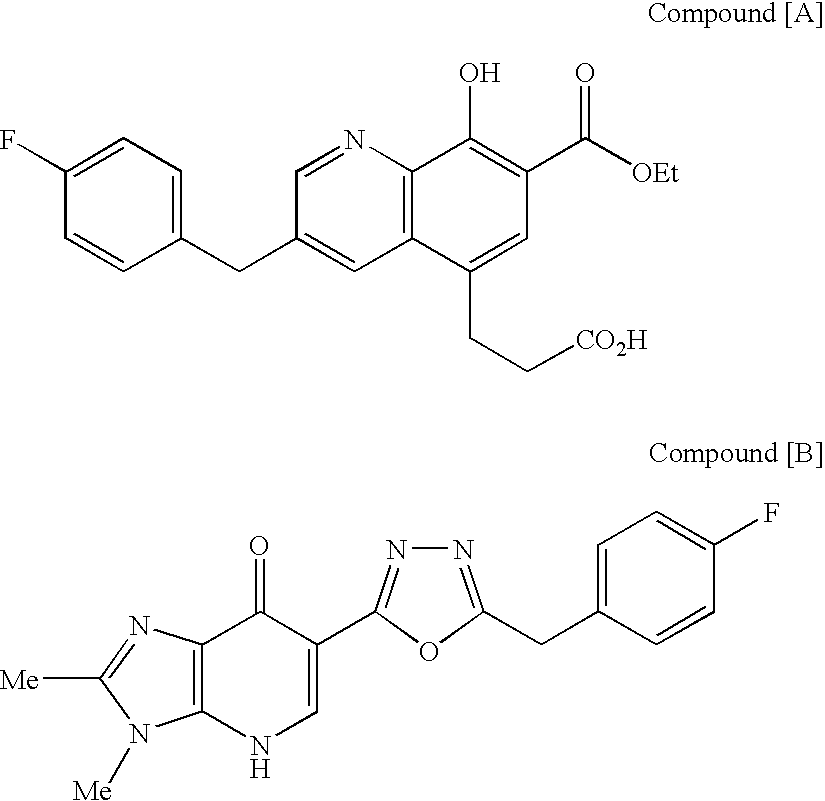
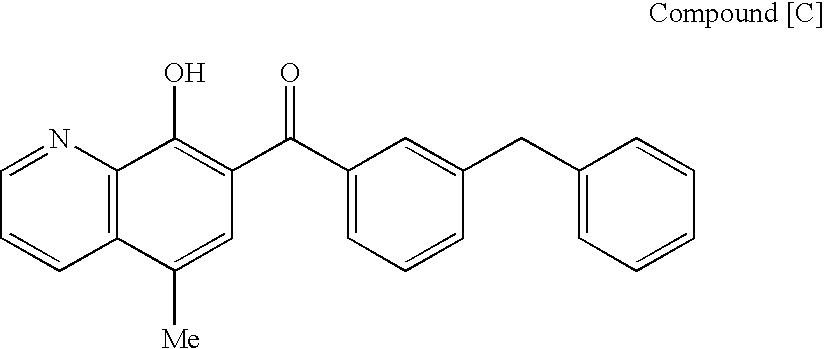
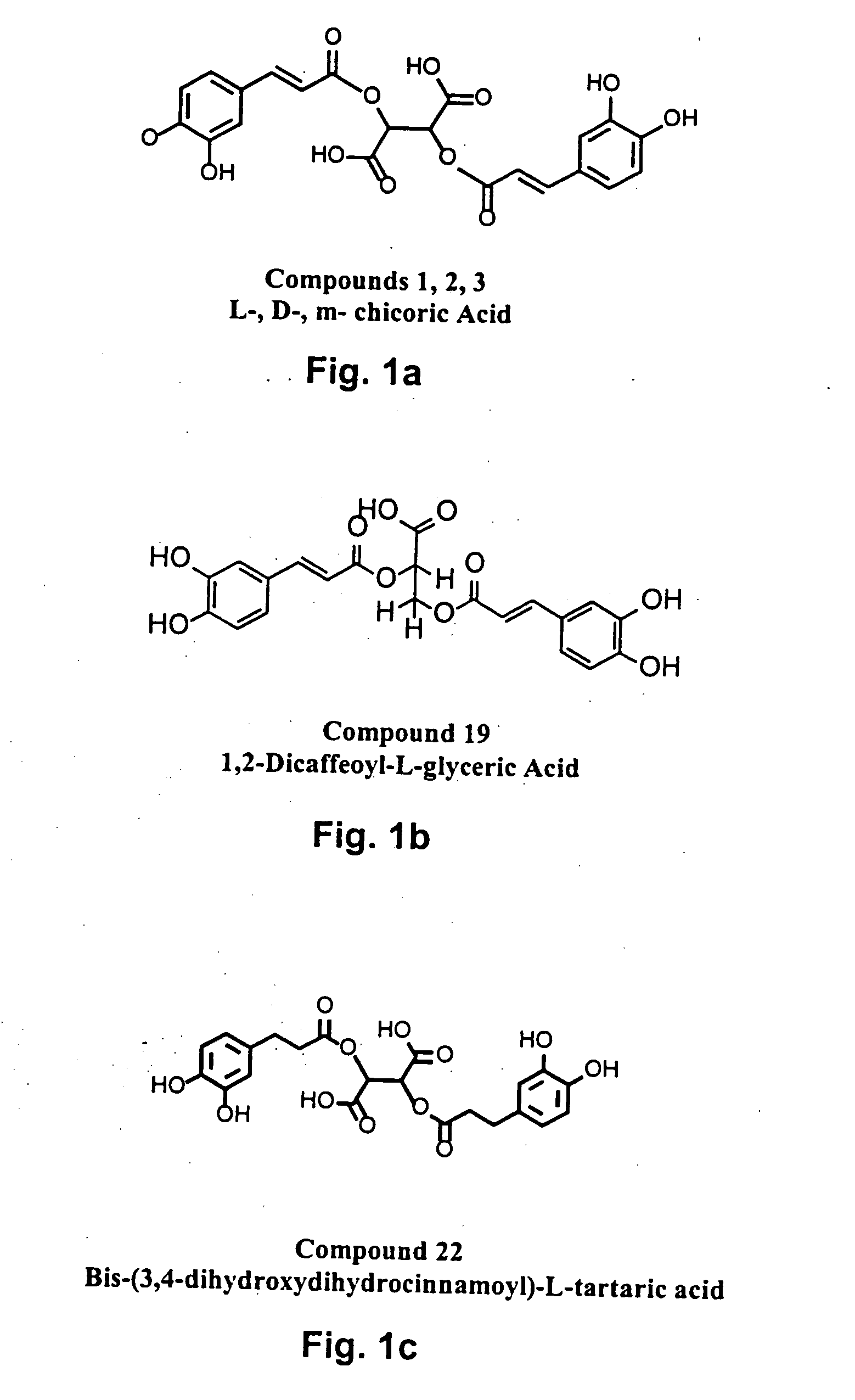
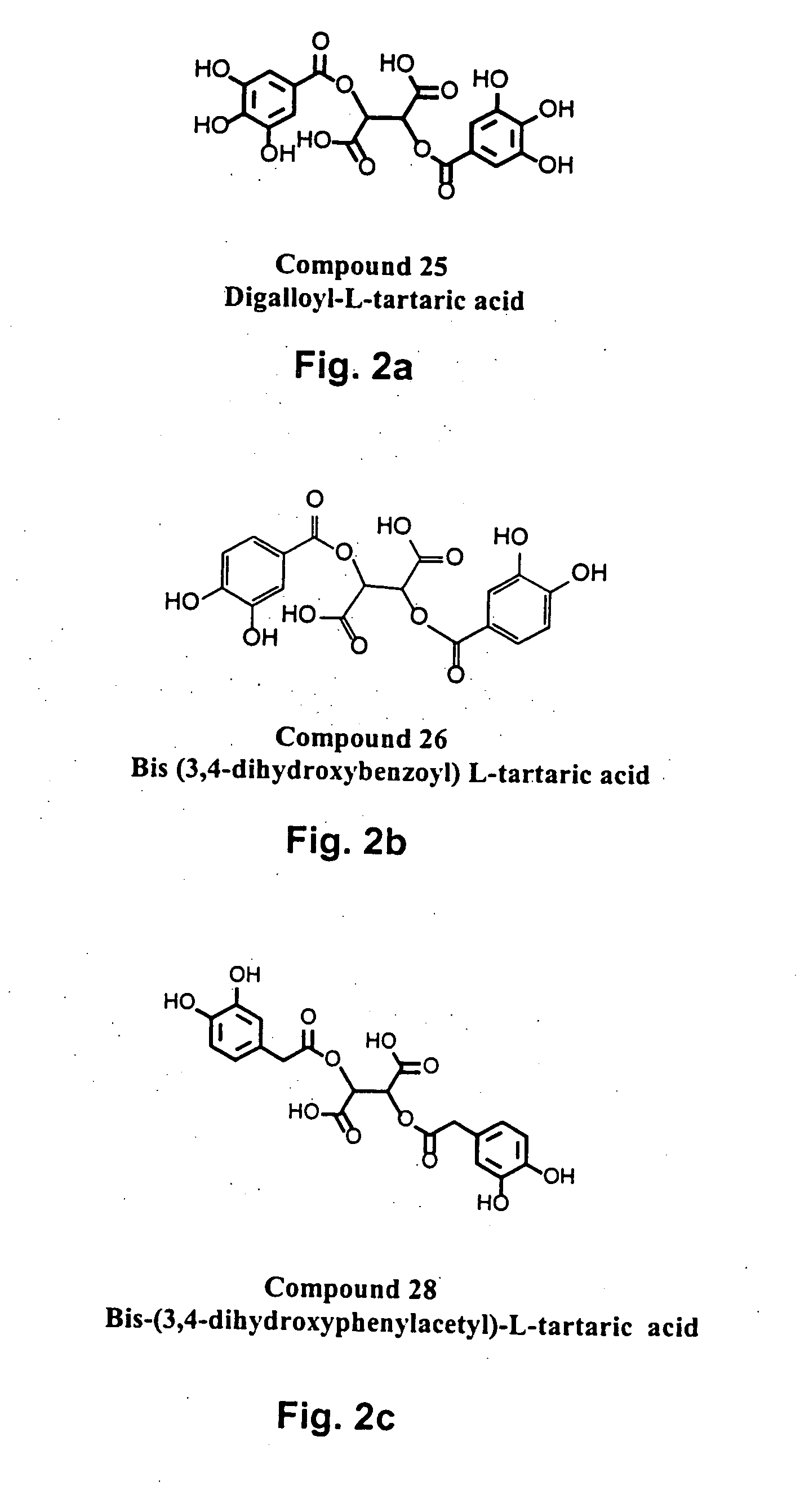
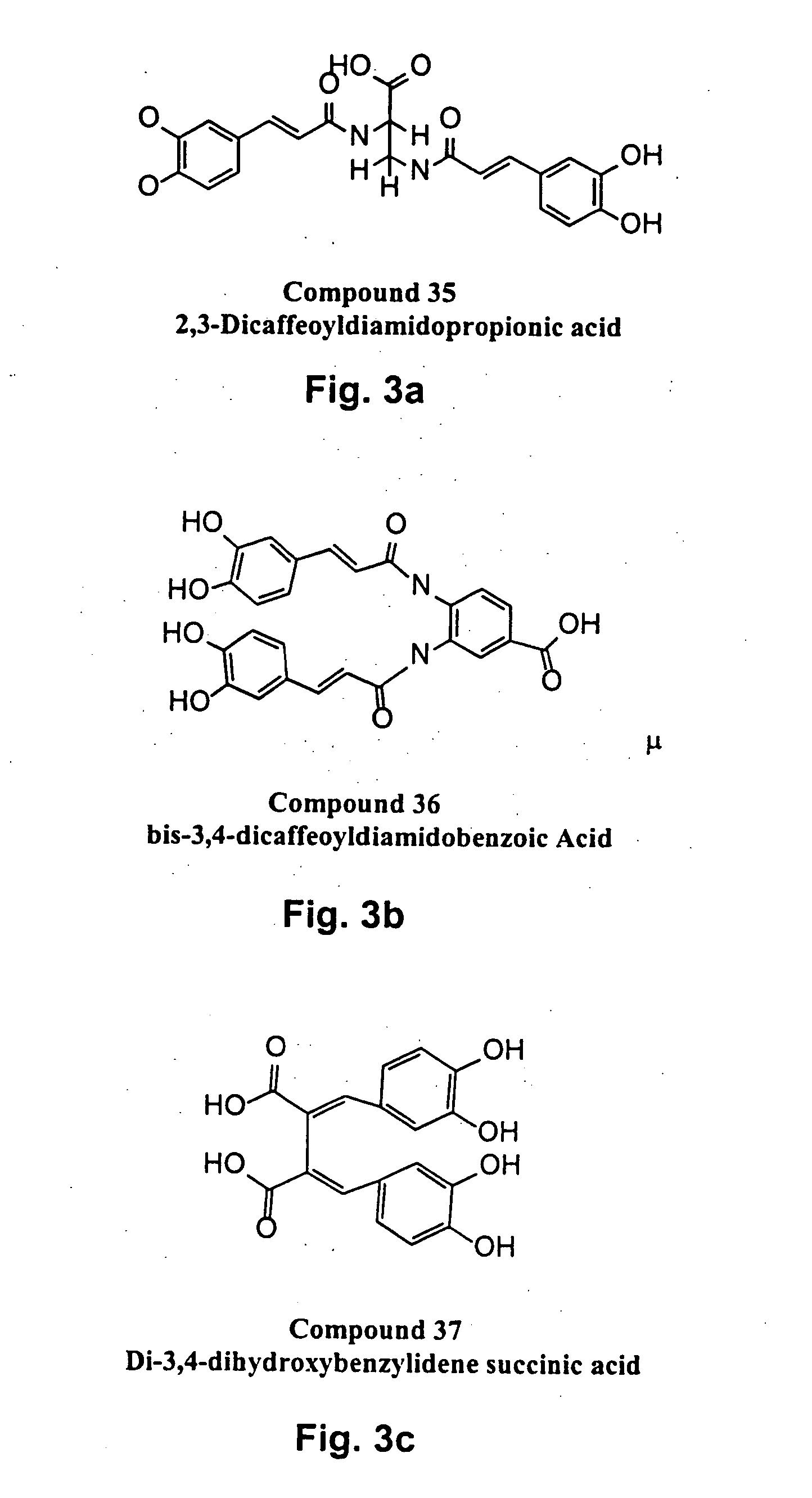
The present invention includes a group of novel compounds that are demonstrated to potently and selectively inhibit HIV integrase (IN) activity in vitro and to potently inhibit HIV replication in live, cultured cells at non-toxic concentrations. The novel compounds disclosed include 2,3-di(3,4-dihydroxy-dihydroxydihydrocinnamoyl)-L-tartaric acid, 2,3-di-(3,4-dihydroxybenzoyl)-L-tartaric acid, 2,3-di-(3,4-dihydroxyphenylacetyl)-L-tartaric acid, 2,3-di-(3,4,5-trihydroxybenzoyl-L-tartaric acid, 2,3-dicaffeoyldiamidopropionic acid, 1,2,-dicaffeoyl-L-glyceric acid, bis,-3,4-dicaffeoyldiamidobenzoic acid, di-3,4-dihydroxybenzylidene succinic acid, di-3,4-dihydrodihydroxybenzylidine succinic acid, 2,3-dicaffeoyl-L-serine, bis-dicaffeoyl-L-isoserine and 1,4-dicaffeoyl-L-lysine. Tests of integrase inhibitors with 2′,3′-dideoxycytidine, zidovudine and nelfinavir (protease inhibitor) indicated a potent synergy against reverse transcriptase inhibitor resistant virus. The potential benefit from the addition of integrase inhibitors to combination drug therapies is significant.
Owner:ROBINSON W EDWARD JR +2
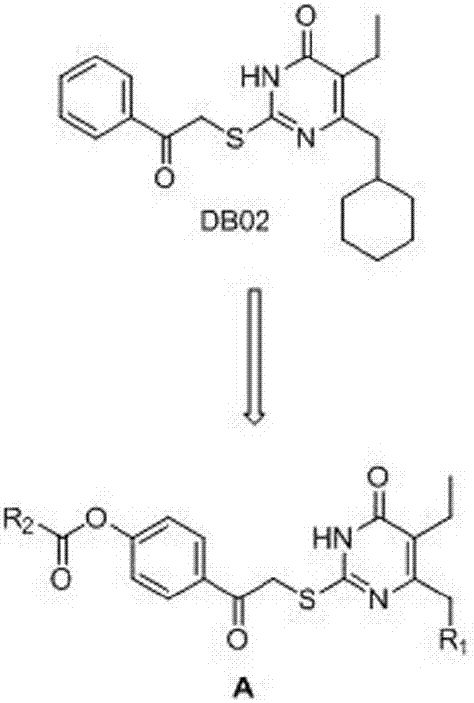
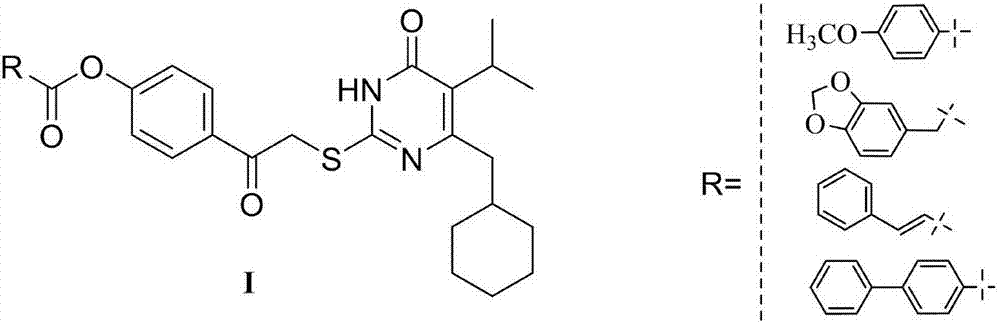
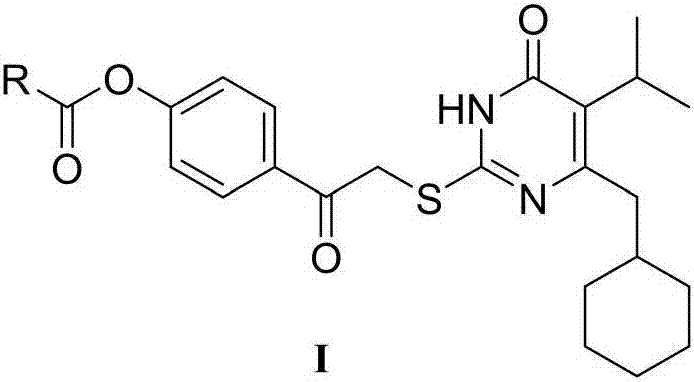
6-cyclohexylpyrimidone HIV reverse transcriptase inhibitor, preparation method and application thereof
InactiveCN106866548ASignificant anti-HIV-1 viral activityLow cytotoxicityOrganic active ingredientsOrganic chemistryNucleoside Reverse Transcriptase InhibitorChemical compound
The invention belongs to the technical field of drugs, and particularly discloses 5-isopropyl-2-(4-hydrocarbyl methanoyl phenyl carbonyl methylsulfonyl)-6-cyclohexylpyrimidone, N-oxide shown as the formula I, a three-dimensional isomer form, a three-dimensional isomer mixture and a pharmaceutically acceptable salt, a hydrous compound and solvent compound, polycrystals and eutectic crystals, a precursor having a same biological function and a derivative. The compound has significant HIV-1 virus resistant activity, is small in toxicity, high in selectivity index and applicable in relevant drugs for treating AIDS. (Shown in the description).
Owner:YUNNAN UNIV +1
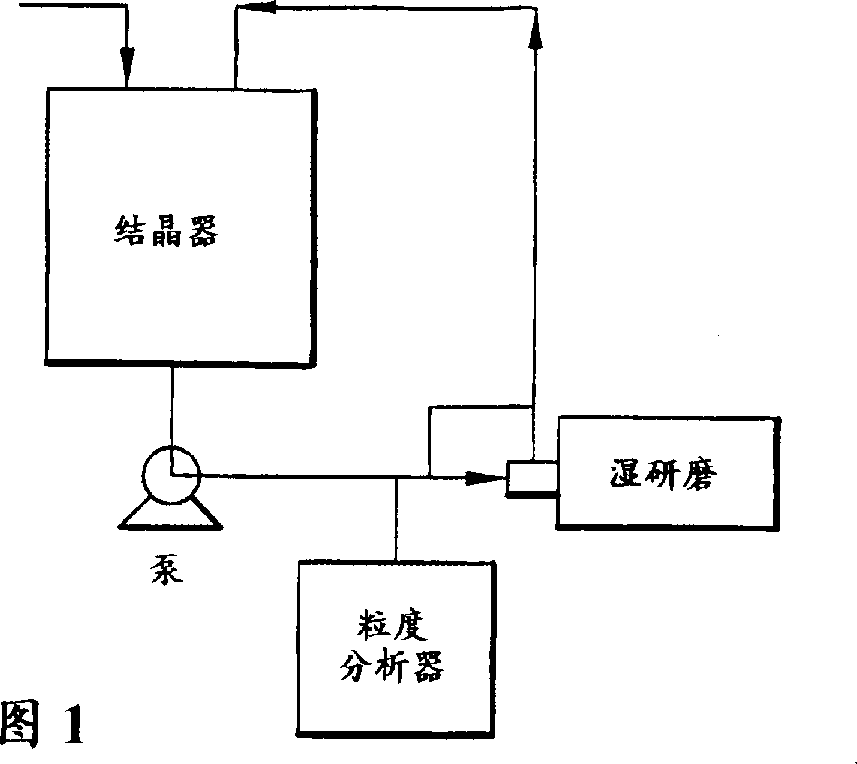
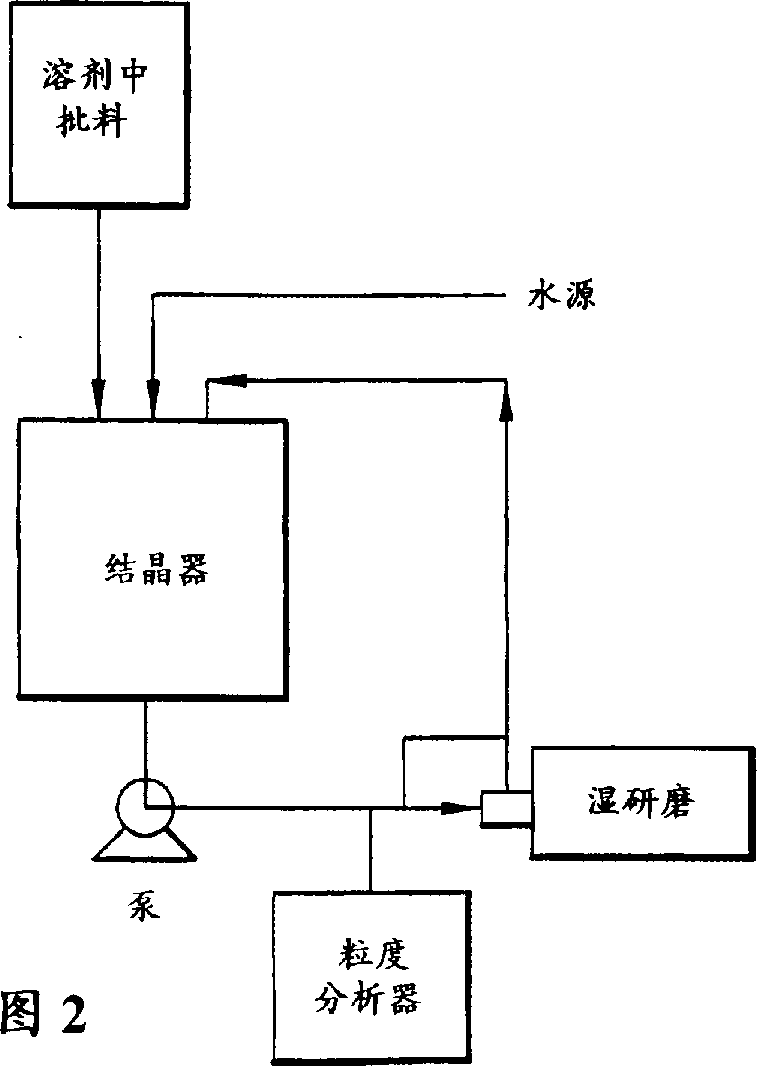
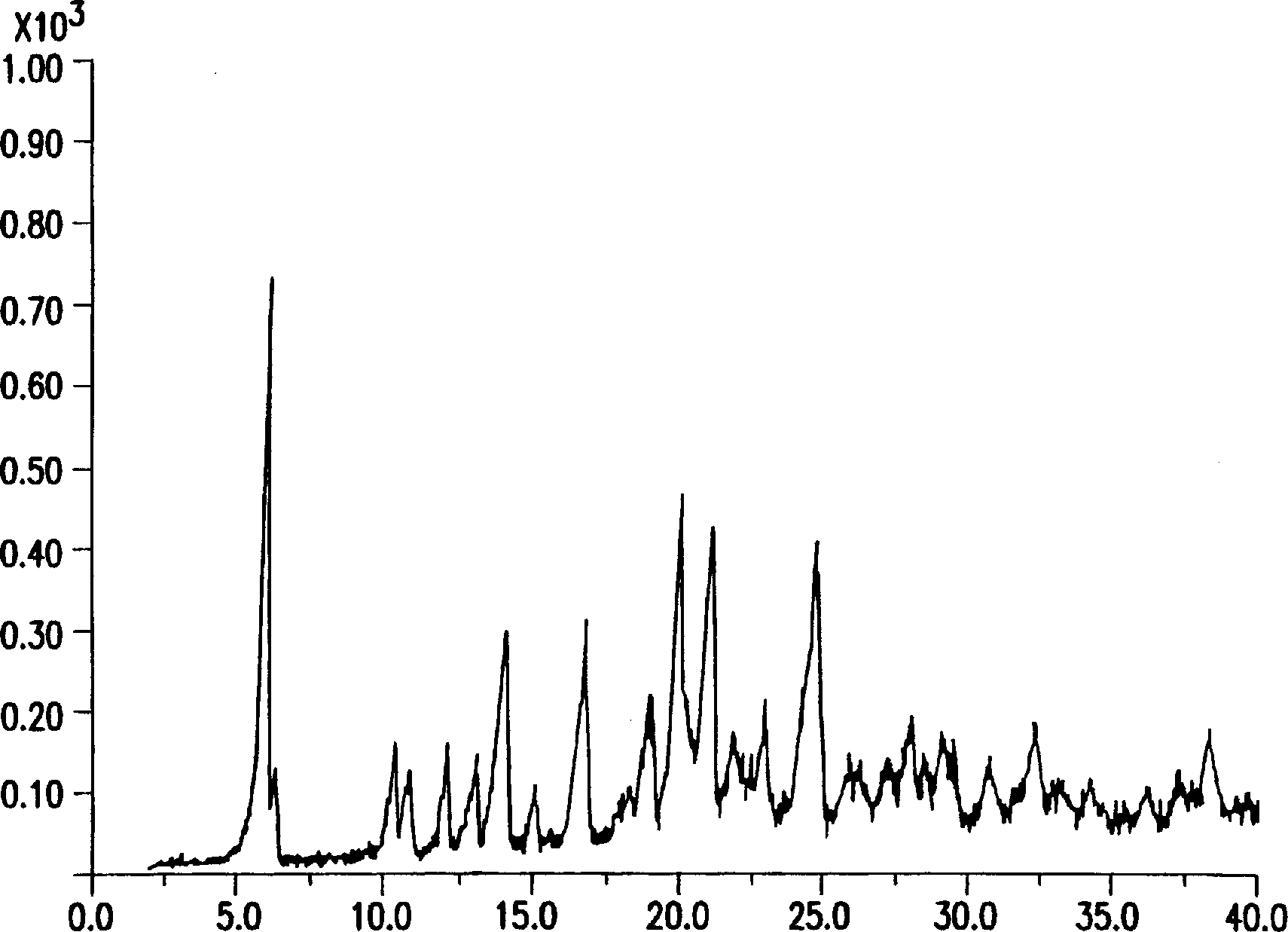
Process for crystallization of reverse transcriptase inhibitor using anti-solvent
InactiveCN1246113APowder deliveryOrganic active ingredientsNucleoside Reverse Transcriptase InhibitorPropanol
The instant invention describes a method for crystallizing (-)-6-chloro-4-cyclopropylethynyl-4-trifluoromethyl-1,4-dihydro-2H-3,1-benzoxazin-2-one from a solvent and anti-solvent solvent system and producing the crystalline product. The desired final crystal form, Form I, can be produced when using methanol or ethanol. Form II is isolated from 2-propanol and can be converted to the desired crystal form at low drying temperatures, such as between about a temperature of 40 DEG C and 50 DEG C.
Owner:SCHERING AG
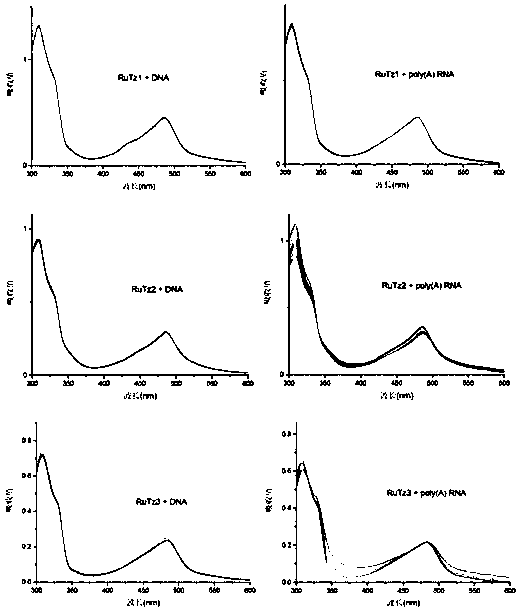
Preparation method for terpyridine pyridinium complex and application thereof in reverse transcriptase inhibition
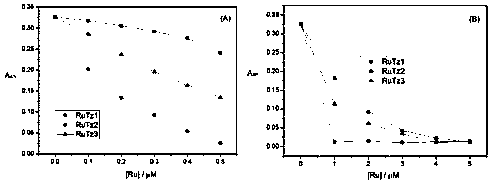
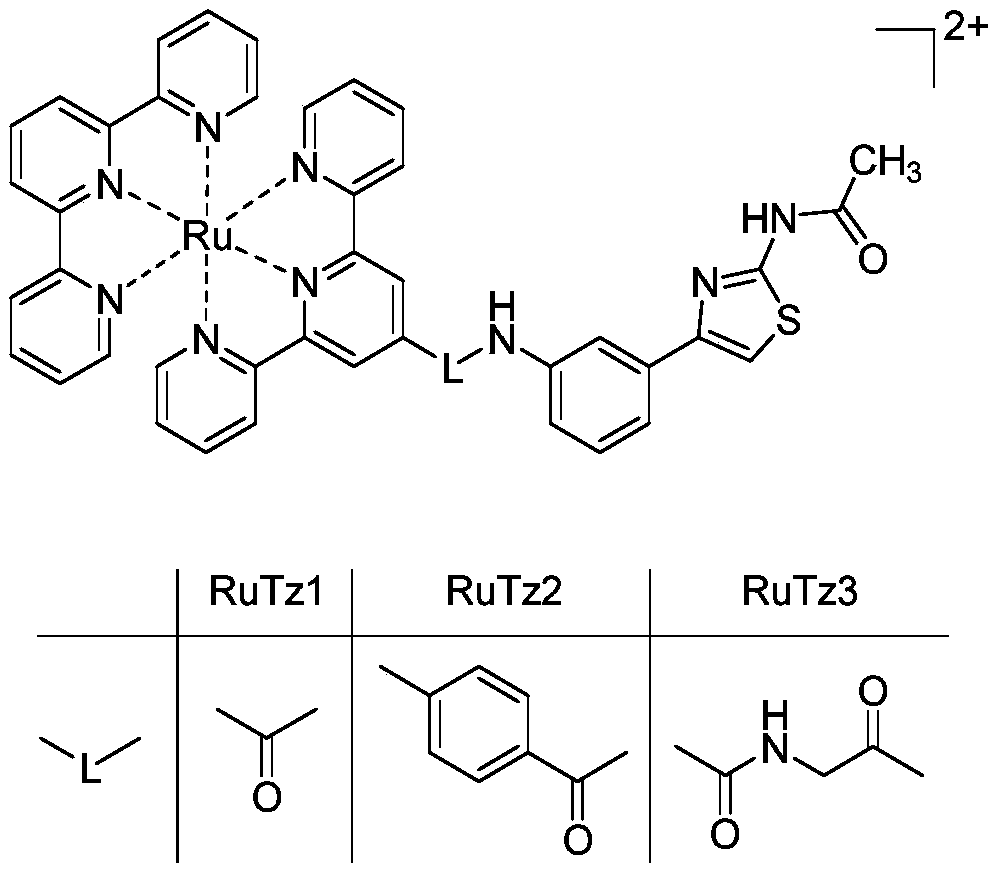
InactiveCN109096339AStable structureGood spectral propertiesRuthenium organic compoundsMaterial analysis by electric/magnetic meansSolubilityNucleoside Reverse Transcriptase Inhibitor
The invention belongs to the field of research and development of HIV inhibitors, and discloses a preparation method for a terpyridine pyridinium (II) complex and application thereof in HIV reverse transcriptase inhibition. The structure of a cationic moiety of the terpyridine pyridinium (II) complex is as shown in a formula I. A preparation process for the terpyridine pyridinium (II) complex is optimized, the raw material cost is low, and the reaction time is short. The obtained complex is high in purity and yield and has good water solubility and excellent spectral properties. The terpyridine pyridinium (II) complex has the capability of selective binding to a TAR region on HIV RNA, and can block the reverse transcription process of viral RNA by reverse transcriptase and inhibit the replication of viral RNA. The terpyridine pyridinium (II) complex is a highly affinitive HIV RNA selective binding reagent and a highly active HIV reverse transcriptase inhibitor, and is an HIV drug having a great application potential.
Owner:YUNNAN UNIV
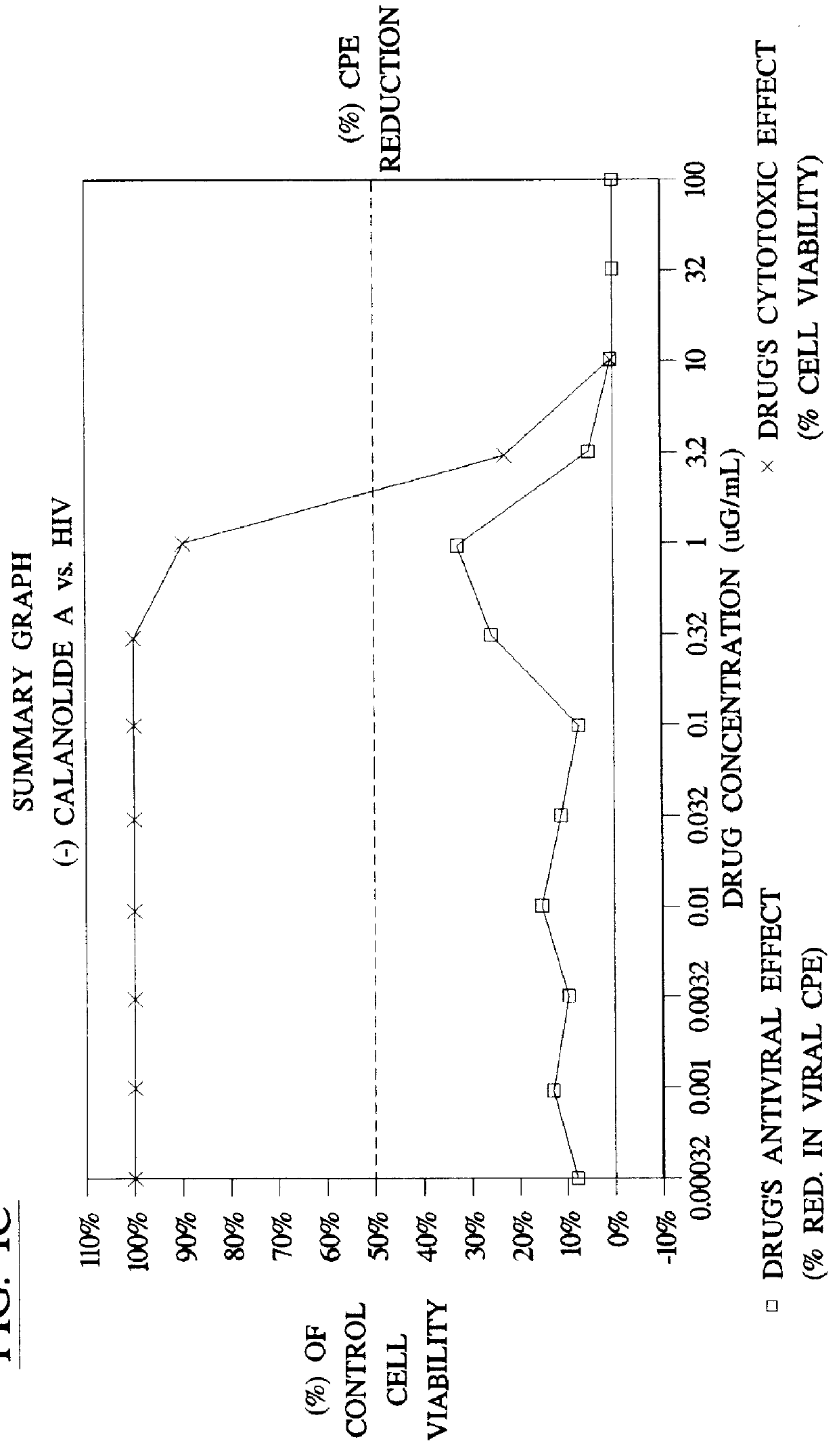
Method for the preparation of (+/-)-calanolide A and intermediates thereof
InactiveUS6043271APractical and convenientHigh yieldBiocideOrganic chemistryHydrolysisEnzyme inhibitor
A method of preparing (+ / -)-calanolide A, 1, a potent HIV reverse transcriptase inhibitor, from chromene 4 is provided. Useful intermediates for preparing (+ / -)-calanolide A and its derivatives are also provided. According to the disclosed method, chromene 4 intermediate was reacted with acetaldehyde diethyl acetal or paraldehyde in the presence of an acid catalyst with heating, or a two-step reaction including an aldol reaction with acetaldehyde and cyclization either under acidic conditions or neutral Mitsunobu conditions, to produce chromanone 7. Reduction of chromanone 7 with sodium borohydride, in the presence of cerium trichloride, produced (+ / -)-calanolide A. A method for resolving (+ / -)-calanolide A into its optically active forms by a chiral HPLC system or by enzymatic acylation and hydrolysis is also disclosed. Finally, a method for treating or preventing a viral infections using (+ / -)-calanolide or (-)-calanolide is provided.
Owner:SARAWAK MEDICHEM PHARMA
Composition and methods used during Anti-hiv treatment
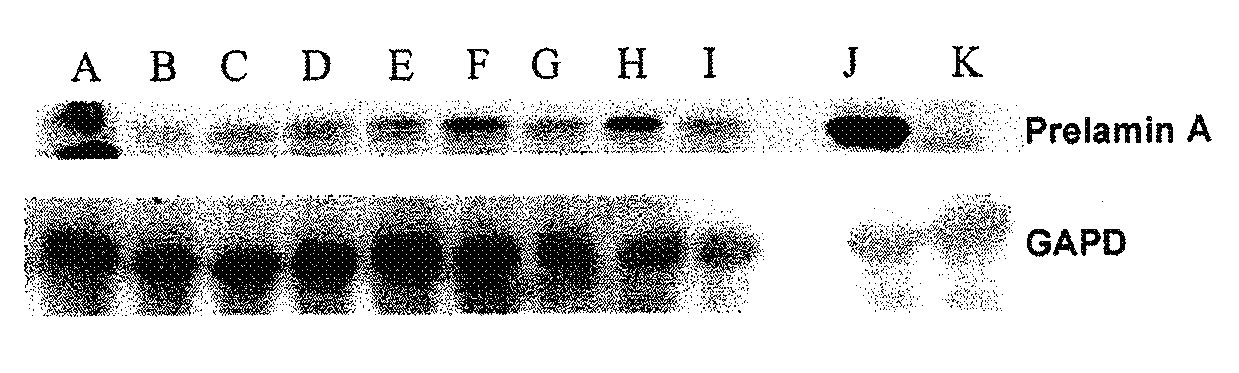
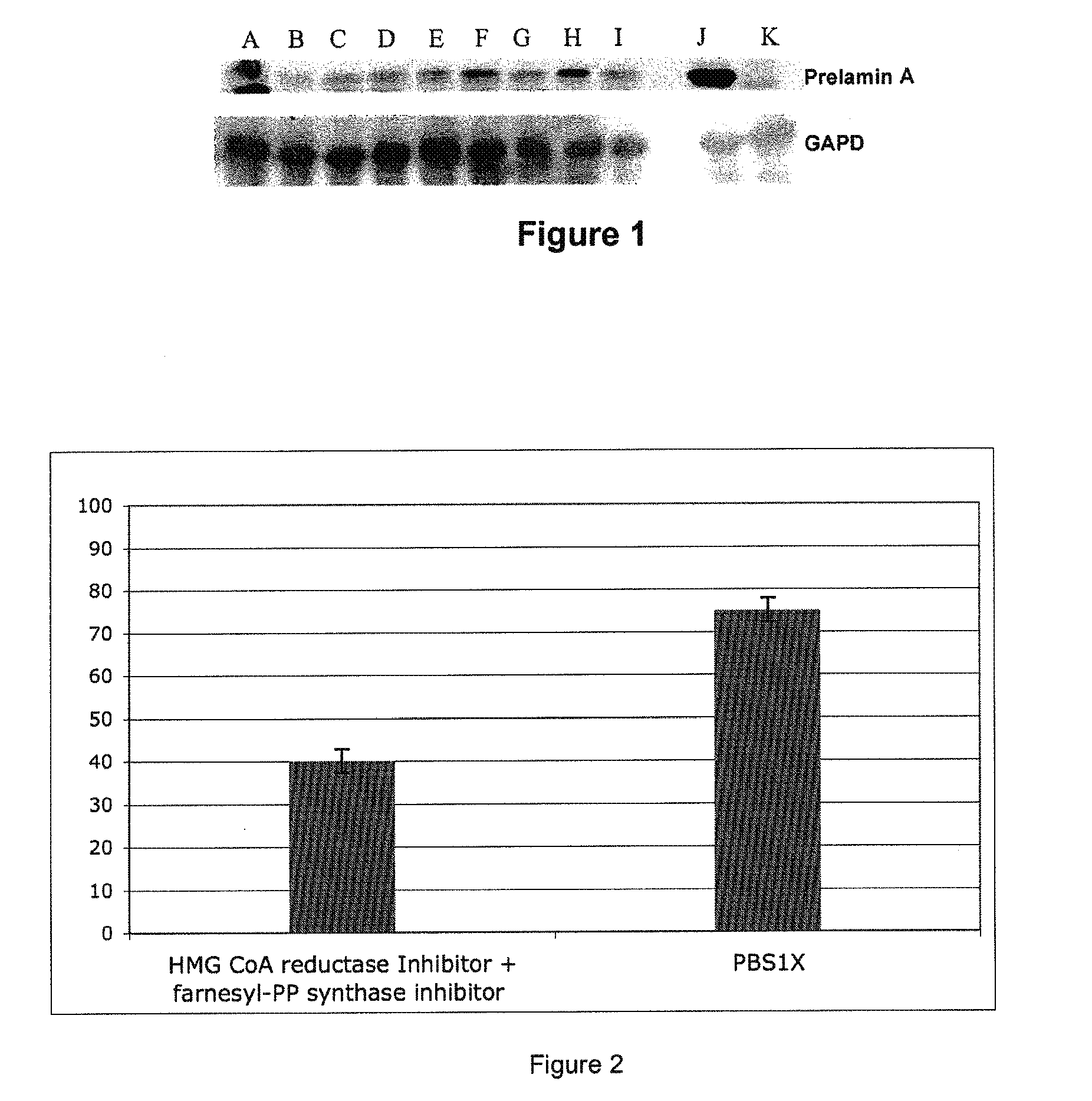
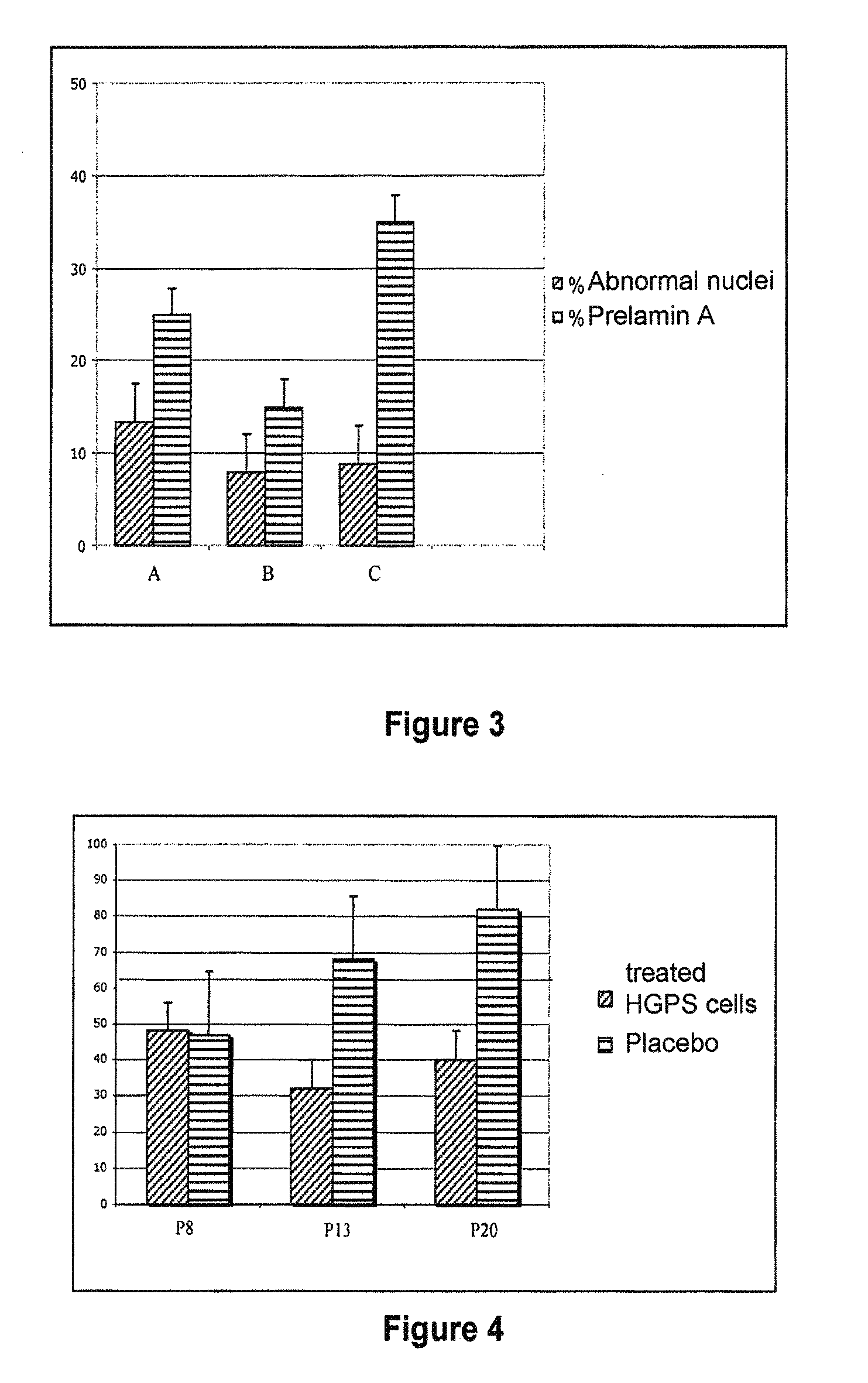
InactiveUS20110046091A1Restoration of the normal phenotypeSymptoms improvedBiocideMetabolism disorderPremature agingSide effect
This invention relates to a composition comprising an anti-HIV treatment and a treatment for side effects of said anti-HIV treatment in an HIV-infected patient. This invention is, for example, very useful in the treatment of side effects caused by certain anti-HIV treatments, for example premature aging and lipodystrophy, which can be caused by protease inhibitors or reverse transcriptase inhibitors. The composition of this invention includes at least one hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor, at least one farnesyl-pyrophosphate synthase inhibitor, and at least one anti-HIV agent. One of the processes for treating an HIV-infected patient includes, in any order, the following steps: (i) administration of a mixture including at least one hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor and at least one farnesyl-pyrophosphate synthase inhibitor and (ii) administration of an anti-HIV agent, in which the administrations are concomitant, successive or alternative.
Owner:UNIV DAIX MARSEILLE
Method for treatment of HIV infection
InactiveUS20060134646A1Readily observable inflammatory responseEliminate side effectsPeptide/protein ingredientsViral antigen ingredientsVacciniaAnti-hiv drugs
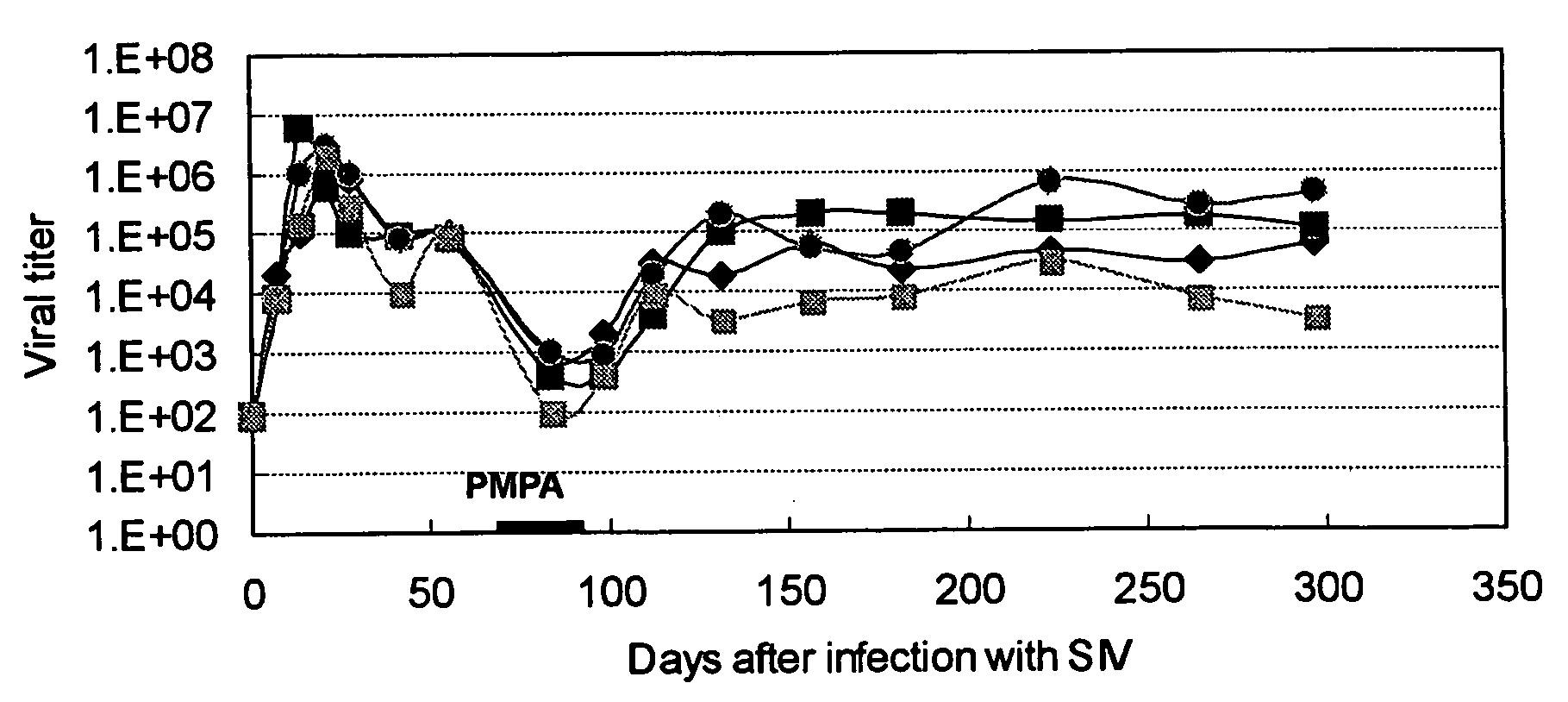
A method for treatment of HIV infection includes administering at least one anti-HIV drug, such as a reverse transcriptase inhibitor, to a patient in need of such treatment and administering an extract from inflammatory tissue inoculated with vaccinia virus to the patient following the administration of the at least one anti-HIV drug. The extract maintains suppressive action on HIV replication, even if the administration of the anti-HIV drug is terminated.
Owner:NIPPON ZOKI PHARM CO LTD
Compositions and methods for treating retinal degradation
The present disclosure relates to compositions and methods for treating retinal damage and / or retinal degradation. More specifically, this disclosure relates to methods for treating degradation of the retinal pigment epithelium by administering compositions comprising a nucleoside and / or a nucleoside or nucleotide reverse transcriptase inhibitor.
Owner:UNIV OF KENTUCKY RES FOUND
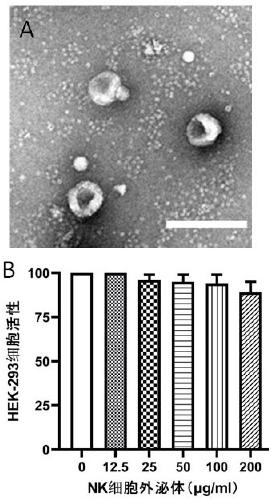
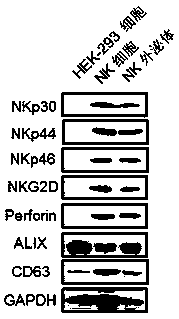
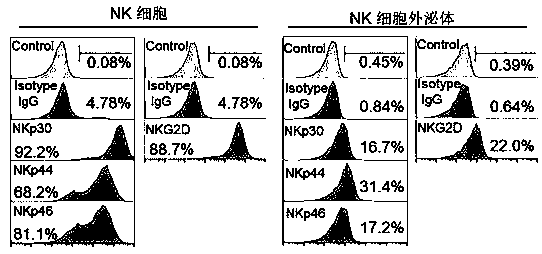
Application of NK cell exosome and related miRNA in preparation of COVID-19 virus inhibitor
ActiveCN111494416ALittle side effectsStrong specificityOrganic active ingredientsAntiviralsReverse transcriptaseNatural Killer Cell Inhibitory Receptors
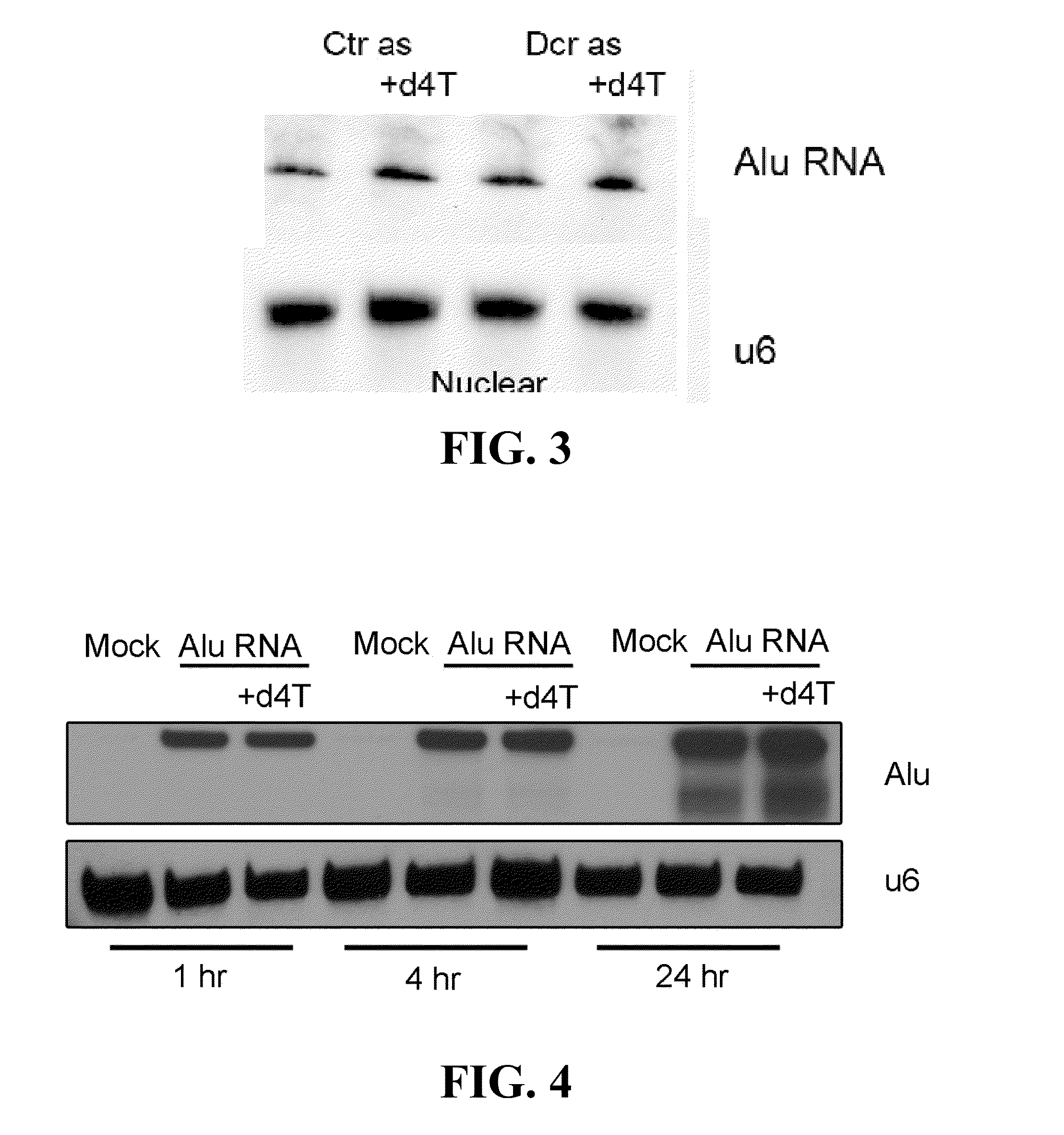
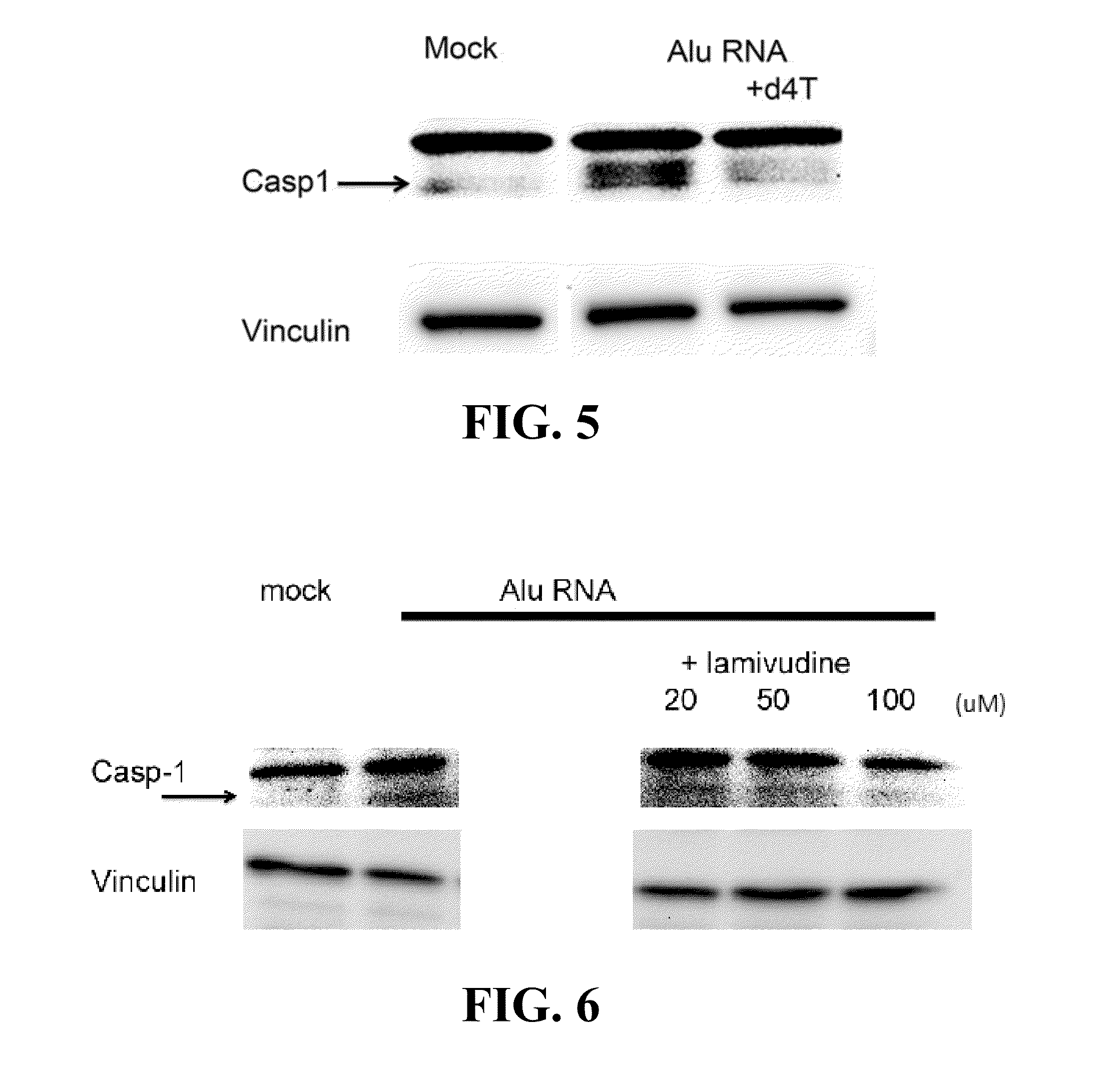
The invention discloses an application of an NK cell exosome (EXO) and related miRNA (micro ribonucleic acid) in inhibition of a COVID-19 virus (new coronavirus), and provides a new thought for inhibition of the COVID-19 virus. The method has the advantages that: (1) the method is different from anti-retroviral inhibitors such as protease inhibitors and reverse transcriptase inhibitors, and miRNAhas high specificity and low side effects; (2) the inhibition effect can be enhanced by combining with a conventional treatment scheme; (3) the miRNA can be artificially synthesized, so that the costis low, and large-scale production can be realized; and (4) the compound has a remarkable inhibition effect on the duplication of the COVID-19 virus.
Owner:SHANGHAI JIAKANG BIOLOGICAL ENG CO LTD
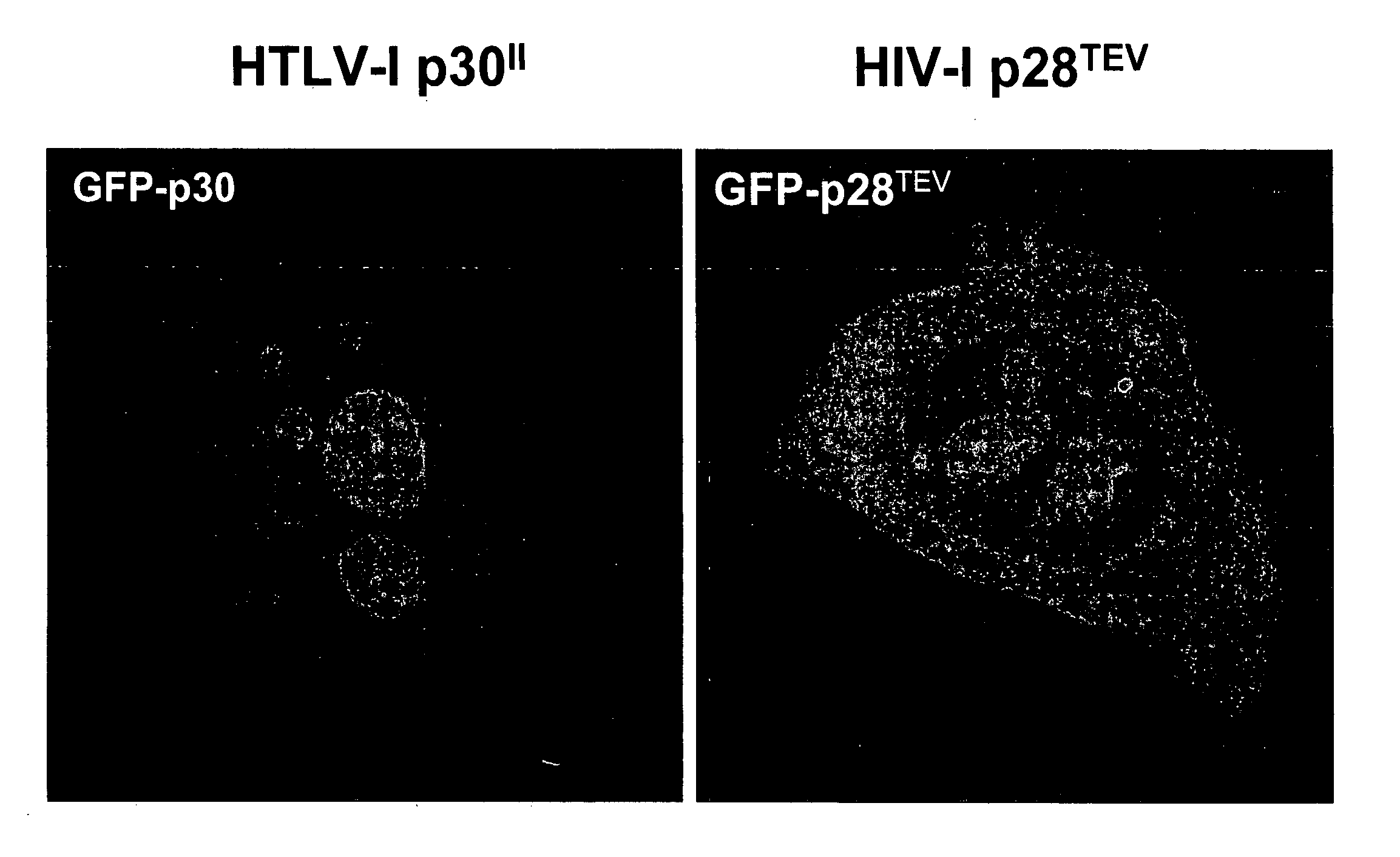
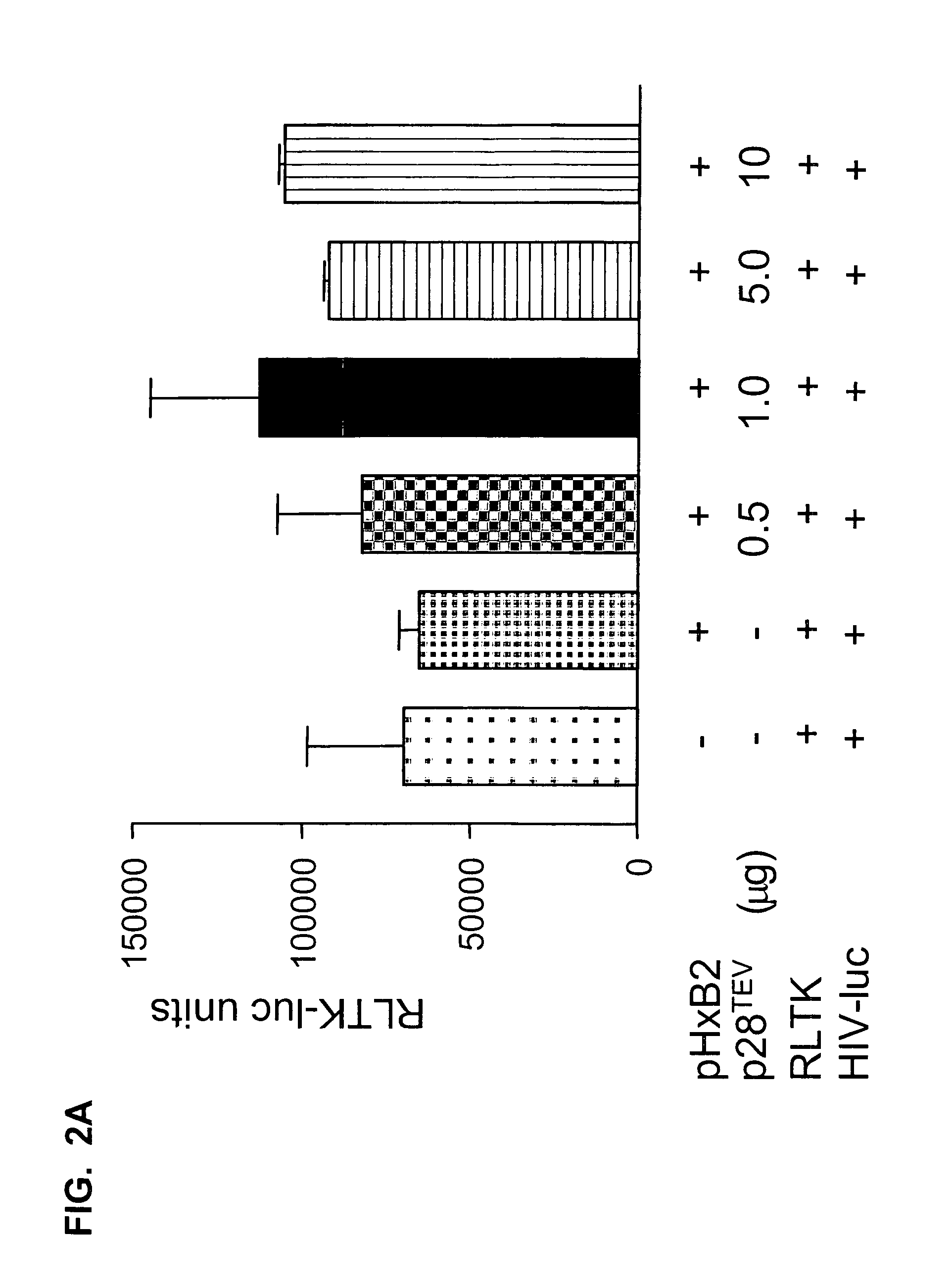
HIV TEV compositions and methods of use
InactiveUS20060205070A1Inhibitory activityInhibit expressionAnimal cellsViral antigen ingredientsReverse transcriptaseNucleotide
The disclosure encompasses p28TEV polypeptide, polynucleotides, variants, and antagonists. p28TEV polypeptides and / or polynucleotides are useful in immunogenic compositions. p28TEV polypeptide antagonists include antagonist antibodies, antisense molecules or siRNA molecules. The antagonists and composition of the disclosure can be administered alone or in combination with other agents useful in the treatment of HIV infection, SIV infection, AIDS, or AIDS-related complex (ARC), including nucleoside, non-nucleoside, and / or reverse transcriptase inhibitors.
Owner:UNITED STATES OF AMERICA
Compositions and methods for treating retinal degradation
Owner:UNIV OF KENTUCKY RES FOUND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-<i>p</i>-amino benozioc acids as HIV reverse transcriptase inhibitors N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-<i>p</i>-amino benozioc acids as HIV reverse transcriptase inhibitors](https://images-eureka.patsnap.com/patent_img/0efc470b-2453-496c-a38c-02b8a1dc5ec7/US07435752-20081014-C00001.png)
![N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-<i>p</i>-amino benozioc acids as HIV reverse transcriptase inhibitors N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-<i>p</i>-amino benozioc acids as HIV reverse transcriptase inhibitors](https://images-eureka.patsnap.com/patent_img/0efc470b-2453-496c-a38c-02b8a1dc5ec7/US07435752-20081014-C00002.png)
![N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-<i>p</i>-amino benozioc acids as HIV reverse transcriptase inhibitors N[S(4-aryl-triazol-3-yl)α-mercaptoacetyl]-<i>p</i>-amino benozioc acids as HIV reverse transcriptase inhibitors](https://images-eureka.patsnap.com/patent_img/0efc470b-2453-496c-a38c-02b8a1dc5ec7/US07435752-20081014-C00003.png)
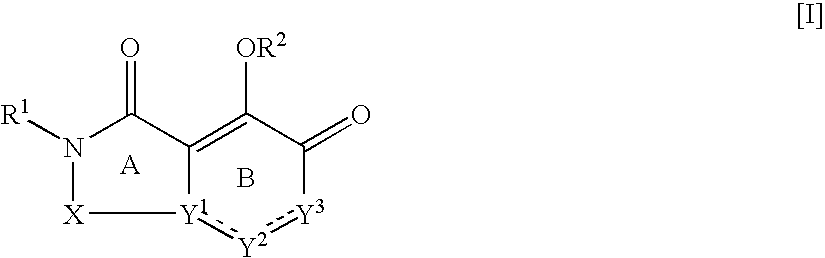
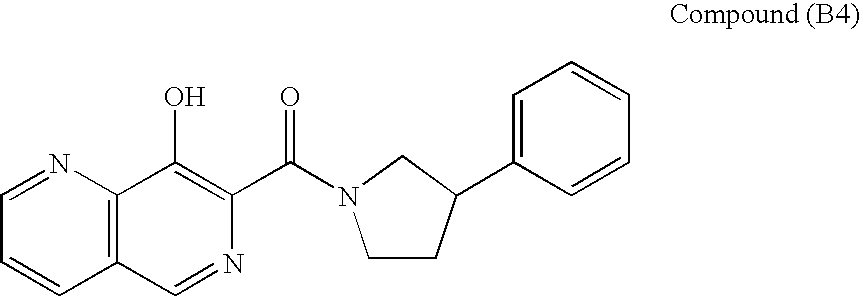
![N[S(4-aryl-triazol-3-yl)alpha-mercaptoacetyl]-p-amino benzoic acids as HIV reverse transcriptase inhibitors N[S(4-aryl-triazol-3-yl)alpha-mercaptoacetyl]-p-amino benzoic acids as HIV reverse transcriptase inhibitors](https://images-eureka.patsnap.com/patent_img/0ddecf1c-7904-4c8e-90a6-e64e68e68c14/US20060270725A1-20061130-C00001.png)
![N[S(4-aryl-triazol-3-yl)alpha-mercaptoacetyl]-p-amino benzoic acids as HIV reverse transcriptase inhibitors N[S(4-aryl-triazol-3-yl)alpha-mercaptoacetyl]-p-amino benzoic acids as HIV reverse transcriptase inhibitors](https://images-eureka.patsnap.com/patent_img/0ddecf1c-7904-4c8e-90a6-e64e68e68c14/US20060270725A1-20061130-C00002.png)
![N[S(4-aryl-triazol-3-yl)alpha-mercaptoacetyl]-p-amino benzoic acids as HIV reverse transcriptase inhibitors N[S(4-aryl-triazol-3-yl)alpha-mercaptoacetyl]-p-amino benzoic acids as HIV reverse transcriptase inhibitors](https://images-eureka.patsnap.com/patent_img/0ddecf1c-7904-4c8e-90a6-e64e68e68c14/US20060270725A1-20061130-C00003.png)
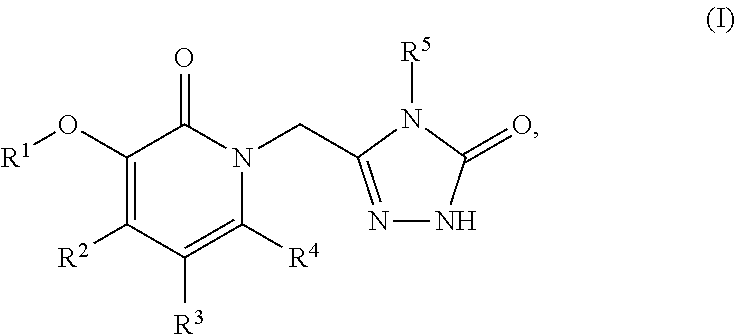
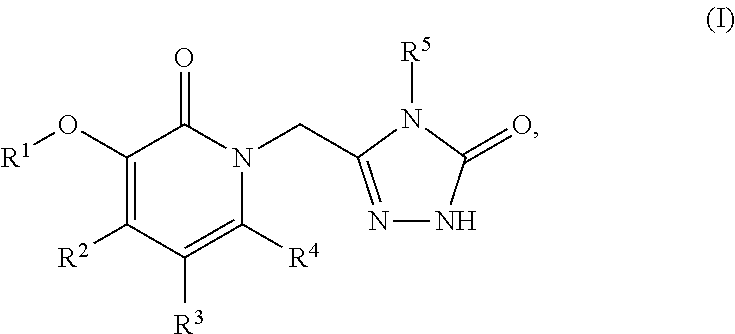
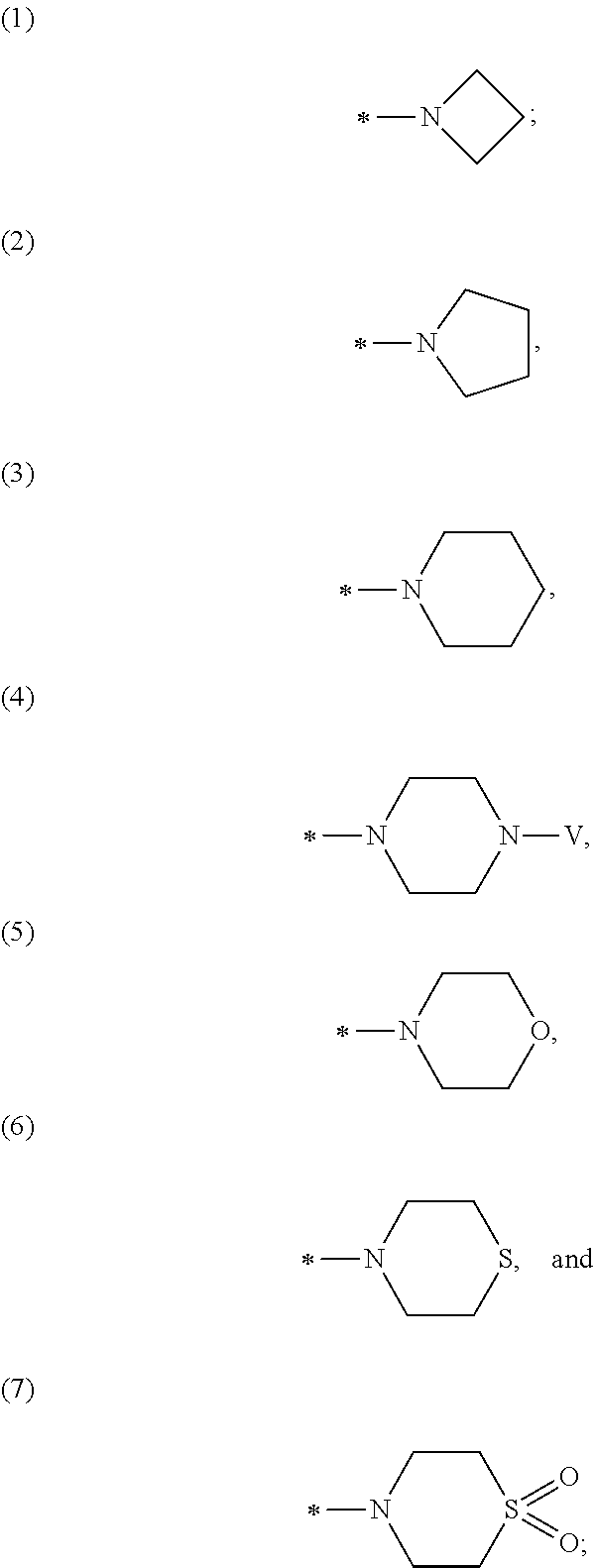
![N[S(4-ARYL-TRIAZOL-3-YL)alpha-MERCAPTOACETYL] -P-AMINO BENZOIC ACIDS AS HIV REVERSE TRANSCRIPTASE INHIBITORS N[S(4-ARYL-TRIAZOL-3-YL)alpha-MERCAPTOACETYL] -P-AMINO BENZOIC ACIDS AS HIV REVERSE TRANSCRIPTASE INHIBITORS](https://images-eureka.patsnap.com/patent_img/964962e7-3fdd-44ed-a453-b52ff6433aa5/US20080319201A1-20081225-C00001.png)
![N[S(4-ARYL-TRIAZOL-3-YL)alpha-MERCAPTOACETYL] -P-AMINO BENZOIC ACIDS AS HIV REVERSE TRANSCRIPTASE INHIBITORS N[S(4-ARYL-TRIAZOL-3-YL)alpha-MERCAPTOACETYL] -P-AMINO BENZOIC ACIDS AS HIV REVERSE TRANSCRIPTASE INHIBITORS](https://images-eureka.patsnap.com/patent_img/964962e7-3fdd-44ed-a453-b52ff6433aa5/US20080319201A1-20081225-C00002.png)
![N[S(4-ARYL-TRIAZOL-3-YL)alpha-MERCAPTOACETYL] -P-AMINO BENZOIC ACIDS AS HIV REVERSE TRANSCRIPTASE INHIBITORS N[S(4-ARYL-TRIAZOL-3-YL)alpha-MERCAPTOACETYL] -P-AMINO BENZOIC ACIDS AS HIV REVERSE TRANSCRIPTASE INHIBITORS](https://images-eureka.patsnap.com/patent_img/964962e7-3fdd-44ed-a453-b52ff6433aa5/US20080319201A1-20081225-C00003.png)