Composition and methods used during Anti-hiv treatment
a technology of composition and treatment, applied in the field of antihiv composition and treatment of hiv-infected patients, can solve the problems of perinatal lethality in mice, modification of the assembly of proteins in the nuclear envelope, and disruption of its functioning
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
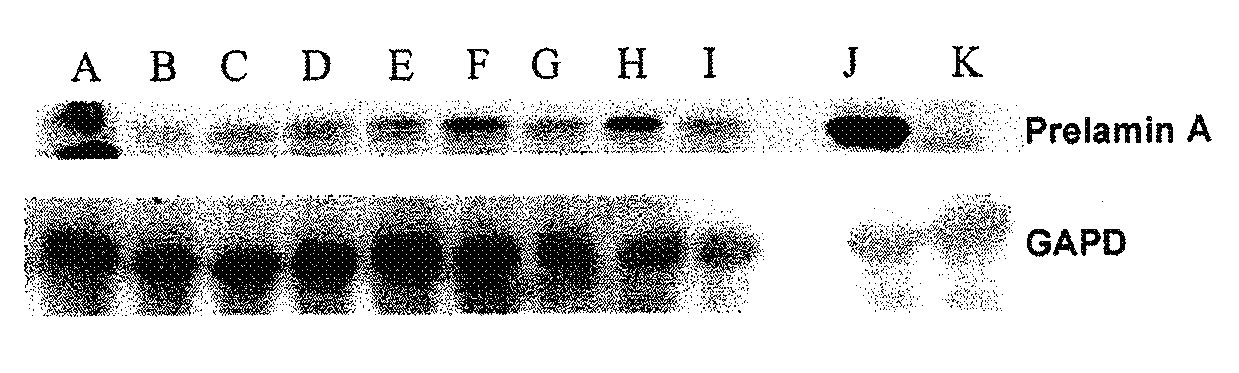
Additive Effect of the Association of a Hydrosoluble Hydroxymethylglutaryl-Coenzyme A (HMG-CoA) Reductase Inhibitor (a Hydrosoluble Statin: Pravastatin) and a Farnesyl-Pyrophosphate Synthase Inhibitor (an Aminobiphosphonate: Zoledronate) on Normal and Progeroid Cell Cultures
A. Protocols
A.1 Cells and Cell Culture
[0212]The cell lines are either control fibroblasts AG16409 from the Coriell Institute or fibroblasts from biopsies of patients with restrictive dermopathy. They are cultivated at 37° C. under 5% CO2 in room P2.
The usual complete culture medium is:[0213]RPMI (Invitrogen) complemented with[0214]Fetal bovine serum 20% (Invitrogen)[0215]L-Glutamine 200 mM (Invitrogen)[0216]Mixture of Penicillin / Streptomycin / Fungizone 1× (Stock 100×, Cambrex)
A.2 Cell Harvesting
[0217]The harvesting of cells is performed by trypsinization as follows (protocol for a large flask, 75 cm2, BD Falcon):[0218]The medium is suctioned;[0219]The cells are washed with 10 ml of PBS 1× (Invitrogen);[0220]5 ml o...
example 2
Effect of a Composition According to the Invention Including a Hydrosoluble Hydroxymethylglutaryl-Coenzyme A (HMG-CoA) Reductase Inhibitor and a Farnesyl-Pyrophosphate Synthase Inhibitor on the Division of Aged Human Fibroblasts and on Young Human Fibroblasts
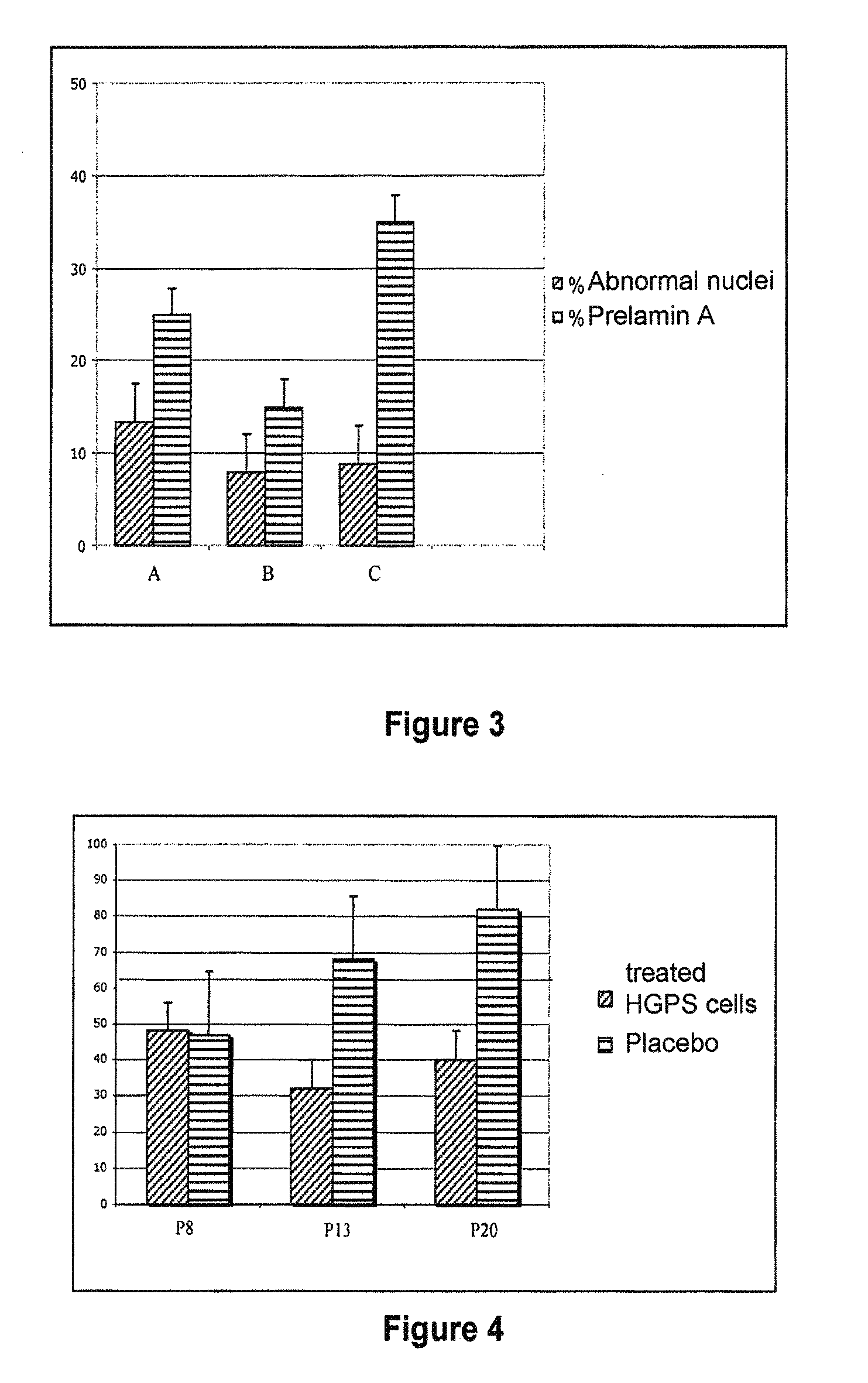
A. Example Objective
[0293]In this example, the evaluation of the in vitro effect of a composition according to the invention including a hydrosoluble hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor and a farnesyl-pyrophosphate synthase inhibitor on the rate of cell division (mitotic index) of fibroblasts was measured. A comparison of the effect of the composition on aged human fibroblasts with respect to young human fibroblasts was also performed. The number of active agents used in this experiment is four, and the products were used in pair combinations. The active agents used are:[0294]A1: Zolendronate[0295]A2: Alendronate[0296]B1: Pravastatin[0297]B2: Simvastatin
[0298]The specific associations that were used in...
example 3
Effect of the Association of a Hydrosoluble Hydroxymethylglutaryl-Coenzyme A (HMG-CoA) Reductase Inhibitor and a Farnesyl-Pyrophosphate Synthase Inhibitor on a Mouse Model Having a Progeroid Syndrome
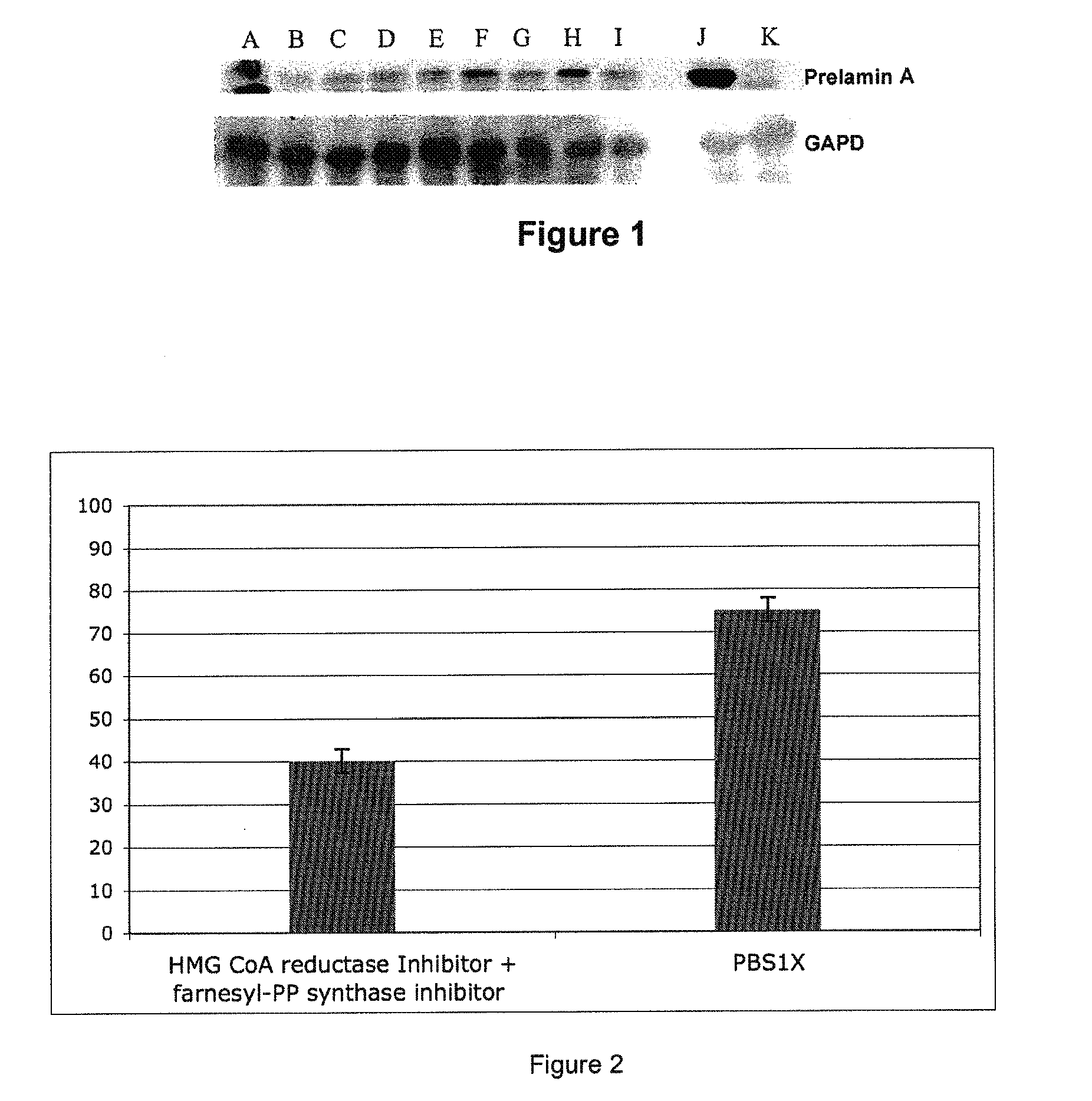
[0312]The Zmpste24− / − KO mice used here are those described in the cited article of Varela & al., 2005 (49). Evidence of efficacy of the association of the two molecules (pravastatin and zoledronate) was reported in collaboration with a Spanish laboratory (C. Lopez-Otin). The efficacy is obtained at combined doses that do not have an effect when the products are used separately, demonstrating an additive effect.
[0313]The two molecules (Zoledronic acid (Zometa (registered trademark)) 100 μg / kg / day and Pravastatin 100 mg / kg / day) were diluted in PBS 1× and injected intraperitoneally, on a daily basis, in 1-month-old mice until their death. The controls are wild mice of the same range, treated with PBS 1× alone.
[0314]The survival of the treated mice was significantly improved, and was maxima...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| treatment time | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com