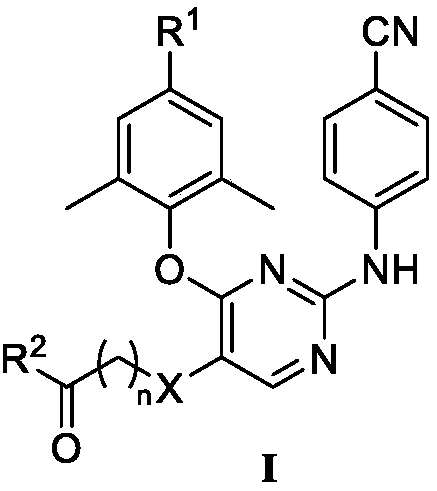
Diarylpyrimidines HIV-1 reverse transcriptase inhibitor as well as preparation method and application thereof
A technology of reverse transcriptase inhibition and diarylpyrimidines, which is applied in the field of organic compound synthesis and pharmaceutical applications, can solve the problems of restricting wide application, poor water solubility, cross-drug resistance, etc., and achieve high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
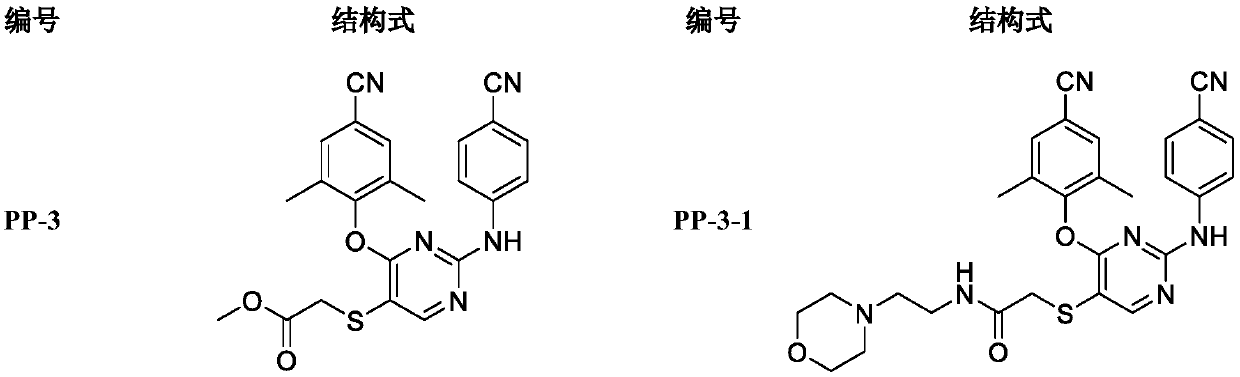
[0039] Example 1: 2-((4-(4-cyano-2,6-dimethylphenoxy)-2-((4-cyanophenyl)amino)pyrimidin-5-yl)mercapto)acetic acid Preparation of methyl ester (PP-3)
[0040]
[0041]Weigh 2,4-dichloropyrimidine (1g, 6.7mmol) and 4-hydroxy-3,5-dimethylbenzonitrile (1.18g, 8mmol) and dissolve in 20mL N,N-dimethylformamide, add K 2 CO 3 (8mmol, 1.10g), stirred overnight at room temperature, filtered and concentrated, dissolved in ethyl acetate, washed 3 times with water, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, and dried in a vacuum oven to obtain a white solid which is compound 6 , yield 86%, melting point: 195-197°C.
[0042] Weigh palladium acetate (0.112g, 0.5mmol) and 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (0.289g, 0.5mmol) and dissolve in 15mL of 1,4-dioxane , and stirred at room temperature for 15 min. Then compound 6 (3.12 g, 0.012 mol) and cesium carbonate (4.89 g, 0.015 mol) were added and stirred for another 15 min. Finally, the raw m...
Embodiment 2
[0047] Example 2: 3-((4-(4-cyano-2,6-dimethylphenoxy)-2-((4-cyanophenyl)amino)pyrimidin-5-yl)mercapto)propane Preparation of acid methyl ester (PP-4)
[0048]
[0049] Weigh PP-2 (1.00g, 4.55mmol), methyl mercaptopropionate (0.547g, 4.55mmol), tris(dibenzylideneacetone) dipalladium (208mg, 0.227mmol), bis(2-diphenyl Phosphino)ether (245mg, 0.455mmol) and potassium tert-butoxide (561mg, 5.00mmol) were dissolved in toluene (20mL), heated to 100°C under nitrogen protection and stirred for 3h. After cooling the reaction mixture to room temperature, the mixture was filtered through celite, and the filtrate was concentrated under reduced pressure. Dichloromethane (80mL) was dissolved, washed with water (20mL×3), washed with saturated brine (20mL), dried over anhydrous sodium sulfate, filtered and separated by silica gel column chromatography to obtain the target compound 3-((4-(4-cyano- 2,6-Dimethylphenoxy)-2-((4-cyanophenyl)amino)pyrimidin-5-yl)mercapto)propanoic acid methyl e...
Embodiment 3
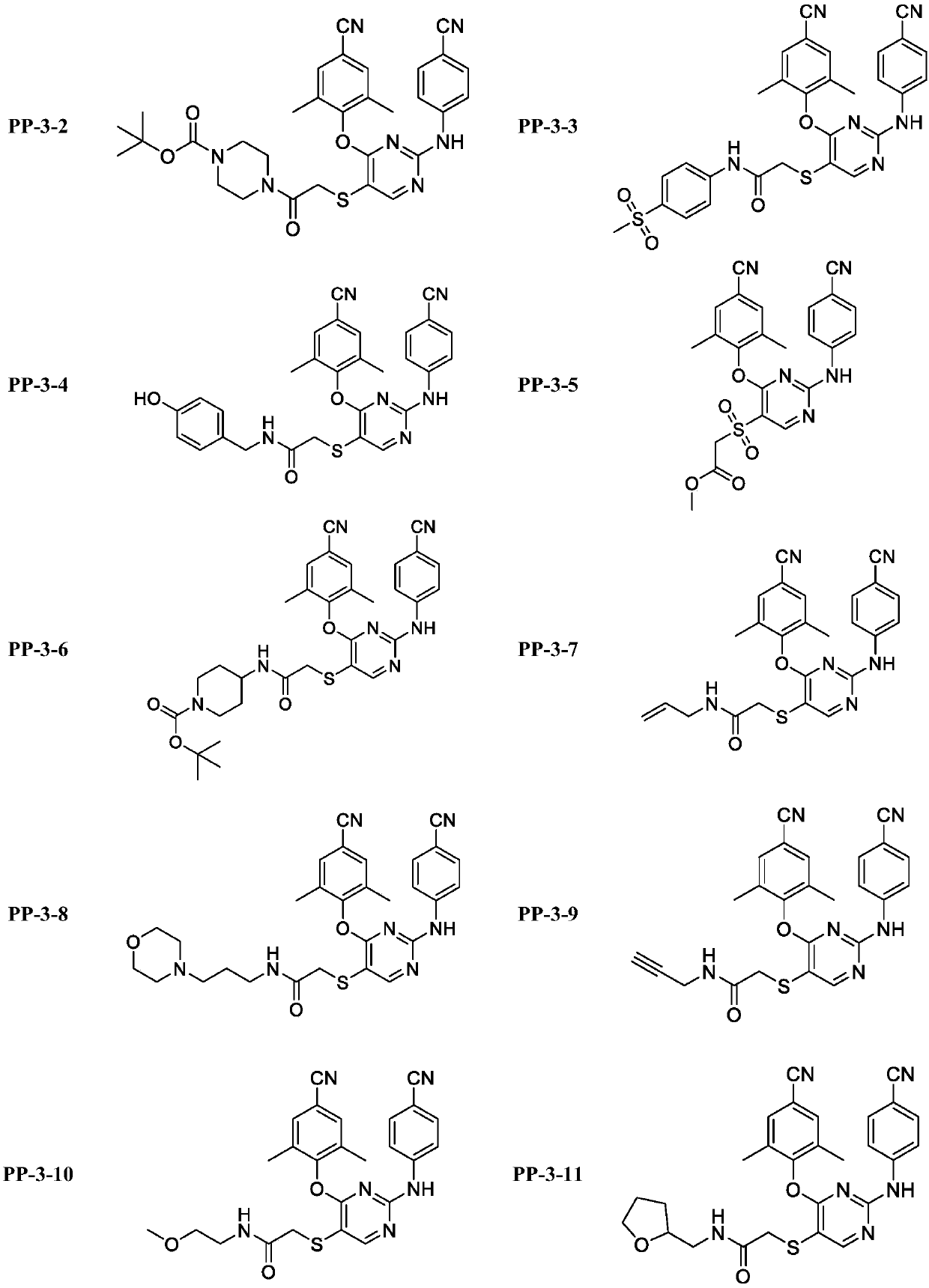
[0052] Example 3: 2-((4-(4-cyano-2,6-dimethylphenoxy)-2-((4-cyanophenyl)amino)pyrimidin-5-yl)sulfonyl) Preparation of methyl acetate (PP-3-5)
[0053]
[0054] Weigh 2-((4-(4-cyano-2,6-dimethylphenoxy)-2-((4-cyanophenyl)amino)pyrimidin-5-yl)thio)acetic acid methyl The ester (100 mg, 0.22 mmol) was dissolved in dichloromethane (10 mL), m-chloroperoxybenzoic acid (132.5 mg, 0.77 mmol) was added and stirred overnight at room temperature. The reaction solution was extracted with dichloromethane (50 mL), washed with saturated sodium bisulfite (30 mL), water (2×20 mL), brine (20 mL). The organic layer was dried over anhydrous sodium sulfate and evaporated to dryness, then recrystallized with ethyl acetate / petroleum ether to obtain the target product 2-((4-(4-cyano-2,6-dimethylphenoxy )-methyl 2-((4-cyanophenyl)amino)pyrimidin-5-yl)sulfonyl)acetate.
[0055] The product is a white solid, yield: 62%, melting point 240-241°C.
[0056] 1 H NMR(400MHz,DMSO-d6):δ11.02(s,1H,NH),8.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com