Method for copolymerizing asymmetrical free-radicals of methacrylate chiral polymer
A technology of methacrylic acid and polymers, which is applied in the field of synthesis of methacrylate chiral polymers, can solve the problems of less research on copolymerization systems, and achieve the effects of simple operation, easy control, and easy realization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
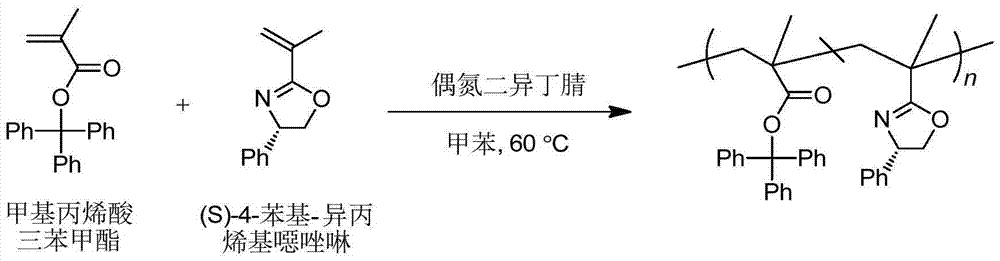
[0027] 1. Take 10.0g trityl chloride and fully dissolve it in toluene at room temperature, then N 2 Under protection, add methacrylic acid (equivalent to the equivalent of trityl chloride, such as figure 1 Shown), then add triethylamine dropwise (the dropping rate should be controlled to be 3 drops / second, 2.3 times the equivalent of trityl chloride), raise the temperature to 80°C, stir and reflux for 3.5h, stop the reaction and cool down, and wash with toluene And filtered, rotary steamed, purified by recrystallization method, and finally obtained pure trityl methacrylate monomer with a yield of 58%.
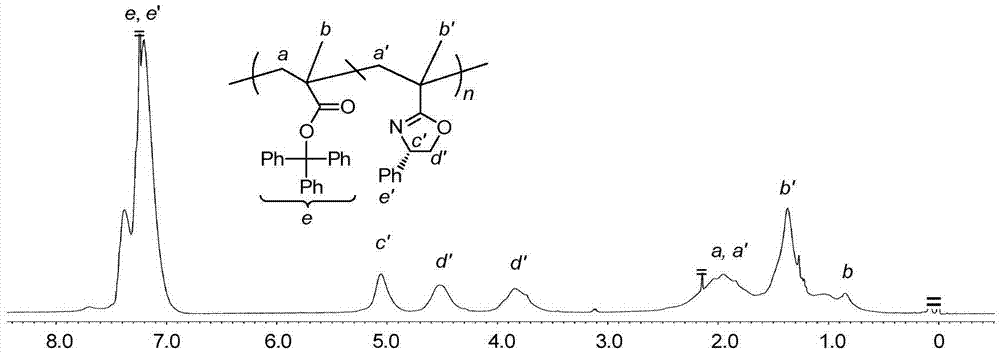
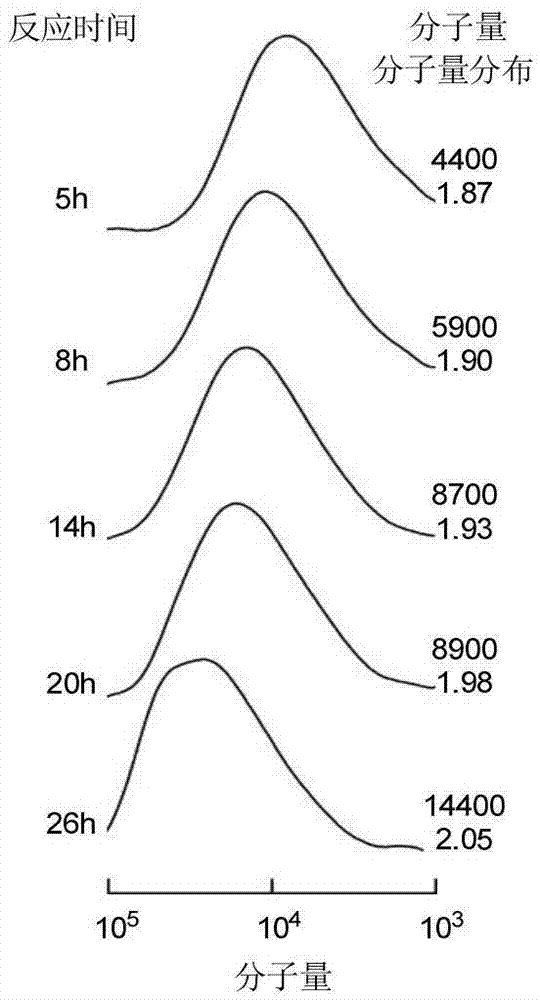
[0028] 2. Add 0.1g trityl methacrylate monomer into the polymerization tube, N 2 Add toluene under protection, stir until fully dissolved, and seal it for later use. Get a 25mL two-necked flask to configure azobisisobutyronitrile (AIBN) initiator solution, N 2Under protection, 0.08 g of azobisisobutyronitrile (AIBN) and 5 mL of toluene were sequentially added, and stirred u...
specific Embodiment approach 2
[0032] 1. Take 10.0g trityl chloride and fully dissolve it in toluene at room temperature, then N 2 Under protection, add methacrylic acid (equivalent to the equivalent of trityl chloride, such as figure 1 Shown), then add triethylamine dropwise (the dropping rate should be controlled to be 2 drops / second, 2.0 times the equivalent of trityl chloride), heat up to 80°C, stir and reflux for 4h, stop the reaction and cool down, wash with toluene and Filtration, rotary evaporation, and purification by recrystallization, finally obtained pure trityl methacrylate monomer with a yield of 61%.
[0033] 2. Add 0.1g trityl methacrylate monomer into the polymerization tube, N 2 Add toluene under protection, stir until fully dissolved, and seal it for later use. Get a 25mL two-necked flask to configure azobisisobutyronitrile (AIBN) initiator solution, N 2 Under protection, 0.08 g of azobisisobutyronitrile (AIBN) and 5 mL of toluene were sequentially added, and stirred until fully disso...
specific Embodiment approach 3
[0037] 1. Take 10.0g trityl chloride and fully dissolve it in toluene at room temperature, then N 2 Under protection, add methacrylic acid (equivalent to the equivalent of trityl chloride, such as figure 1 Shown), then add triethylamine dropwise (the dropping rate should be controlled to be 1 drop / second, 2.0 times the equivalent of trityl chloride), heat up to 80°C, stir and reflux for 4h, stop the reaction and cool down, wash with toluene and Filtration, rotary evaporation, and purification by recrystallization, finally obtained pure trityl methacrylate monomer with a yield of 64%.
[0038] 2. Add 0.1g trityl methacrylate monomer into the polymerization tube, N 2 Add toluene under protection, stir until fully dissolved, and seal it for later use. Get a 25mL two-necked flask to configure azobisisobutyronitrile (AIBN) initiator solution, N 2 Under protection, 0.08 g of azobisisobutyronitrile (AIBN) and 5 mL of toluene were sequentially added, and stirred until fully dissol...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com