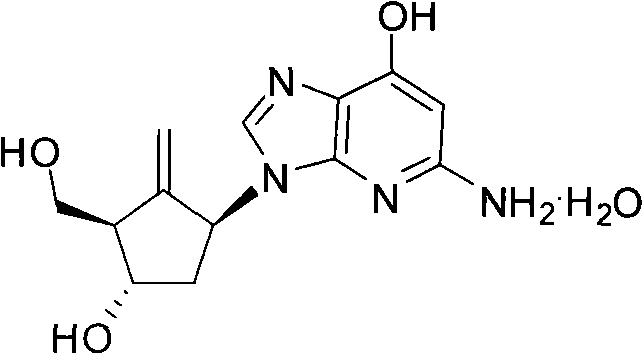
A kind of preparation method of antiviral drug entecavir
An antiviral drug, Entecavir technology, applied in the production of bulk chemicals, organic chemistry, etc., can solve the problems of distance from reaction conditions, difficulty in separation and purification, unstable yield, etc., and achieve low cost, wide source of raw materials, and product purification method simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
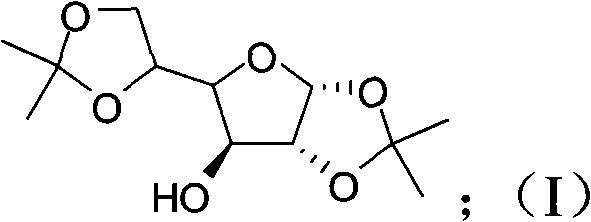
[0045] (1), preparation compound I:
[0046] Feeding:
[0047]
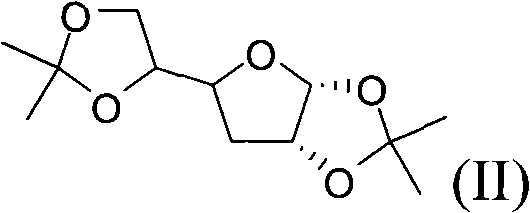
[0048] Reaction formula:
[0049]
[0050] D-glucose compound-I
[0051] reaction process:
[0052] Anhydrous glucose (257g) was stirred and suspended in 5000ml of acetone, and the temperature of the system was cooled to below 5°C in an ice bath, and the concentrated H 2 SO 4 (205.6ml) was placed in the dropping funnel, added dropwise to the stirring system, and the temperature was controlled below 10°C. After the concentrated sulfuric acid was added, the temperature was naturally raised to room temperature and reacted overnight. Cool the system below 5°C with an ice bath, and neutralize it by adding 33.3% NaOH dropwise. The reaction solution was filtered, the filtrate was concentrated by rotary evaporation, and after cooling, the 2 Cl 2 Extraction, H 2 O washes the organic phase, anhydrous Na 2 SO 4 Drying, rotary evaporation, and recrystallization from ethyl acetate and petroleum ether gave com...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com