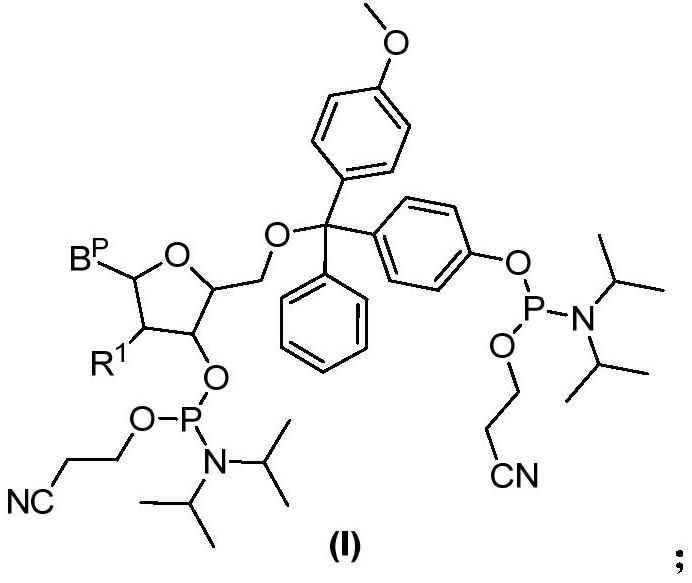
A kind of nucleoside bisphosphoramidite and preparation method thereof
A compound and general formula technology, applied in the field of nucleoside compound synthesis, can solve problems affecting the synthesis efficiency, purification and quality control of oligonucleotide monomers
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0090] Embodiment 1, the synthesis of thymidine bisphosphoramidite
[0091] 1. Synthesis of Compound A
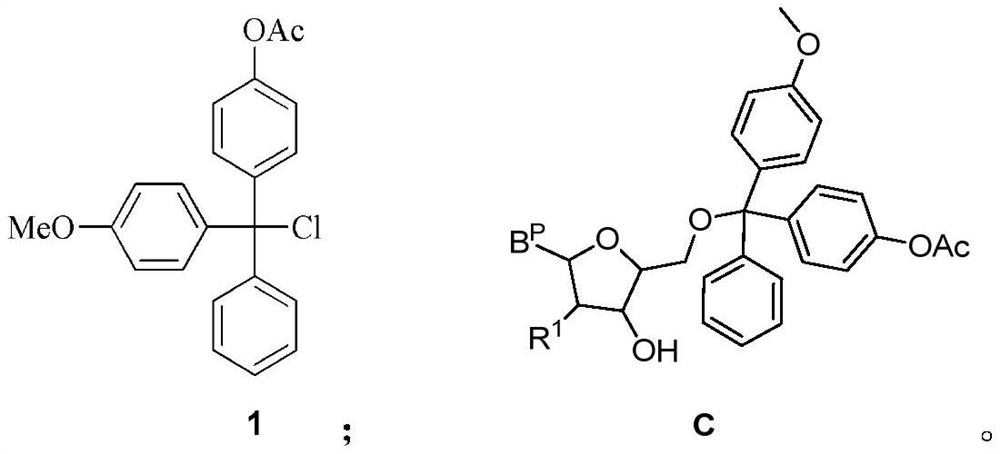
[0092] Weigh 100 g of DMTCl (295.8 mmol) in a dry three-necked flask, add 400 ml of dichloromethane (DCM) to dissolve, and then use argon gas bubbling to drive off the air in the system. After the temperature of the reaction solution was lowered to 0° C. with an ice-water bath, 444 ml of 1M boron tribromide (BBr) was gradually added dropwise to the above system. 3 , 443.7mmol) dichloromethane solution, the dropwise addition time is about 60min. After the dropwise addition, the reaction was stirred at 20±5°C for about 12 hours until the raw material was no longer converted. HPLC monitors the reaction, and if the raw material is <7%, the reaction is stopped; otherwise, the reaction is continued, and samples are taken for HPLC analysis every 50-60 minutes until the raw material is <7%. After the reaction was completed, the reaction solution was concentrated to dryness under...
Embodiment 2
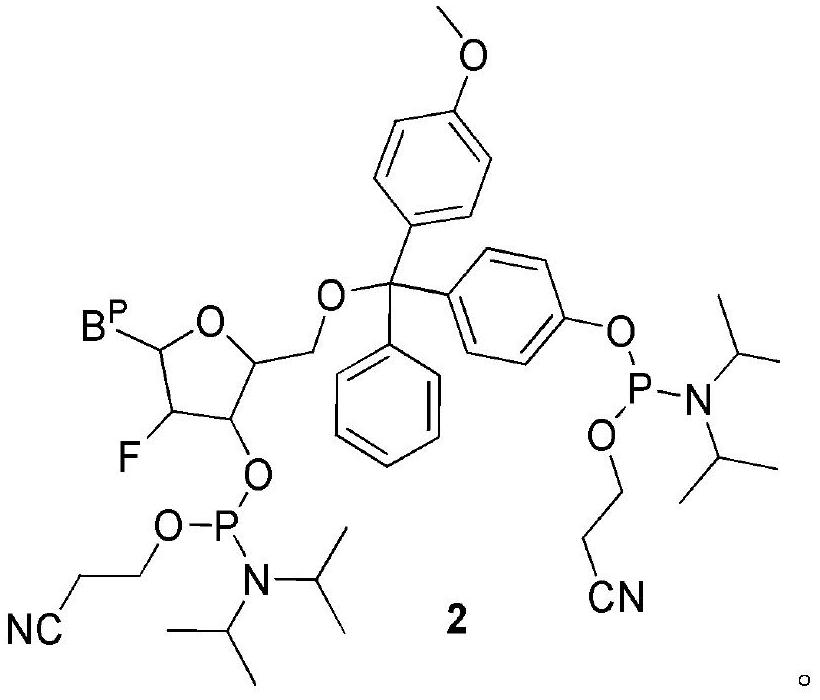
[0105] Embodiment 2, the synthesis of uridine bisphosphoramidite
[0106] Compound 1 was prepared according to the corresponding method in Example 1.
[0107] Synthesis of compound C-2
[0108] Weigh 28.4g of 2-F-uridine (115.4mmol) and 44g of compound 1 (121.2mmol) into a 250ml three-neck flask and stir and dissolve them with 180ml of dry pyridine. After stirring at room temperature for 5 h, TLC (CH 2 Cl 2 : MeOH=95:5, v / v) and HPLC monitors the reaction until the raw material disappears substantially (conversion rate 90%), and stops the reaction. The system was quenched by adding 6 ml of methanol, and concentrated to remove all solvents to obtain a crude product as a pale yellow solid. The crude product was diluted with 600ml of dichloromethane, washed with 600ml of water, and the organic phase was separated and dried with 10g of anhydrous sodium sulfate to obtain 76g of the crude product. The crude product was directly used in the next reaction without purification.
...
Embodiment 3
[0114] Embodiment 3, the synthesis of cytidine bisphosphorimide
[0115] Compound 1 was prepared according to the corresponding method in Example 1.
[0116] Compound 1 was reacted with 4-acetyl-2'-fluoro-dC, and the structure shown in cytidine bisphosphorimide 2c was obtained by referring to the corresponding steps in Example 1. Mass Spectrum: MS (ESI) m / z 976.6 (M+H + ,100).
[0117]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com