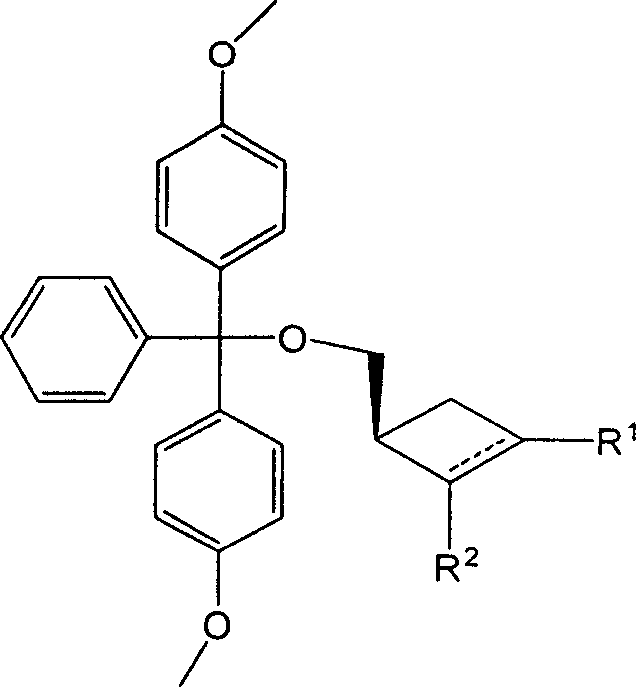
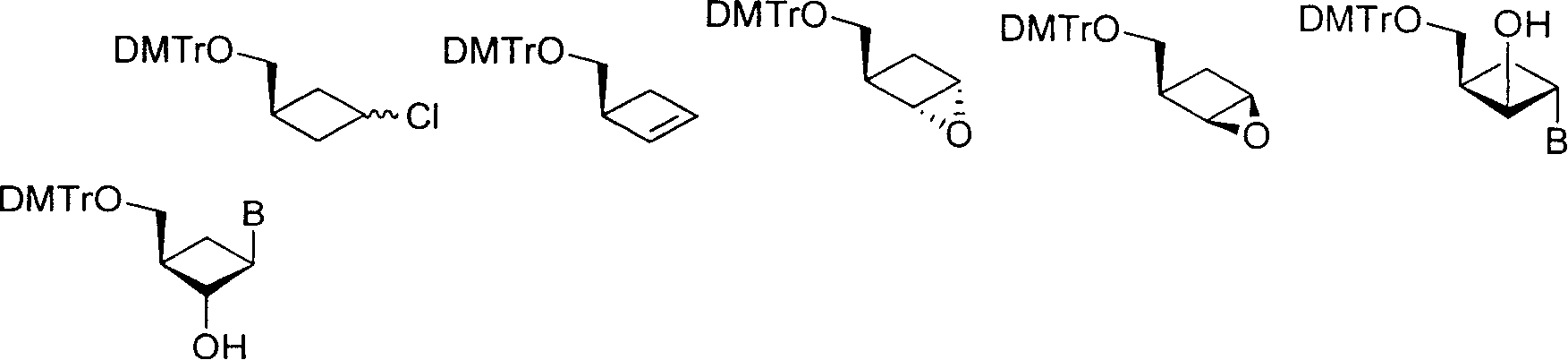
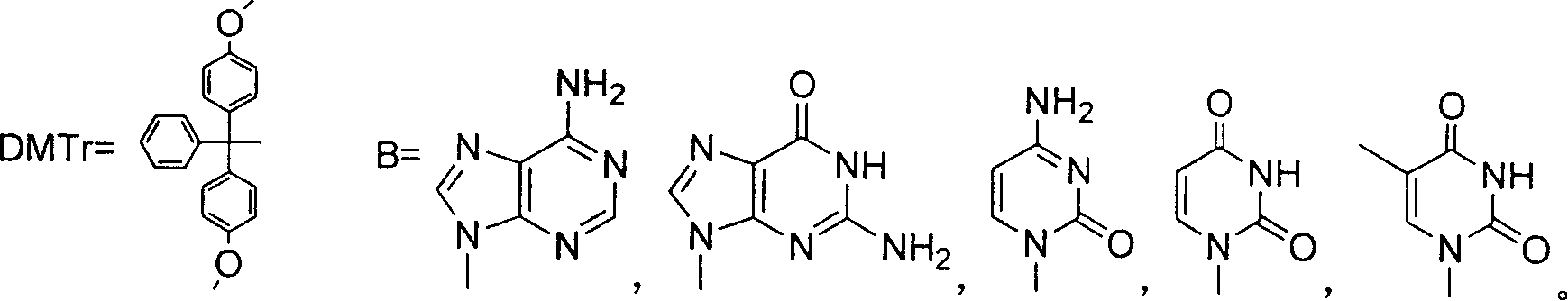
Synthesizing nucleoside analog for antivirus
A synthesis method and product technology, applied in antiviral agents, bulk chemical production, organic chemistry, etc., can solve problems such as ordinary silica gel column separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of compound 9:
[0025] 0.5 g (4 mmol) of raw materials were dissolved in 10 ml of pyridine (potassium hydroxide dried), stirred, and 1.6 g (4.7 mol) of 4,4'-dimethoxytrityl chloride was added. After stirring at room temperature for 3 hours, evaporate the pyridine to dryness, add 30ml of water, and extract with ethyl acetate (25ml×3). The organic phases were combined, washed with saturated brine (25ml×3), and dried over anhydrous sodium sulfate. After filtering and evaporating the solvent, the obtained crude product was subjected to column chromatography (eluent: n-hexane: ethyl acetate = 20:1) to obtain 1.4 g of the product (yield: 94%). 1 HNMR (CDCl 3 ): δ7.44-7.20(m, 9H); 6.85-6.82(m, 4H); 4.74-4.22(m, 1H); 3.79(s, 6H); 3.12-3.15(m, 2H); 2.60-3.00 (m, 2H), 2.05-2.45 (m, 3H).
Embodiment 2
[0027] Preparation of Compound 10:
[0028] Under the protection of argon, add 0.824 g of potassium tert-butoxide into a dry 25 ml three-necked flask, add dry 5 ml of dimethyl sulfoxide, stir, and dropwise add 1.36 g of compound 9 dissolved in 5 ml of dimethyl sulfoxide. Stir overnight. Then the reaction solution was poured into 20ml ice water, the pH value was adjusted to neutral with 5% hydrochloric acid, sodium chloride was added to saturation, extracted with ether (20ml×3), the organic phases were combined, and washed with water (20ml×3) , dried over anhydrous sodium sulfate. After filtering and evaporating the solvent to dryness, the obtained crude product was subjected to column chromatography (eluent: n-hexane:ethyl acetate=20:1), and 1.23 g of the product was obtained (yield: 98%). 1 HNMR (CDCl 3 ): δ7.47-7.20(m, 9H); 6.85-6.80(m, 4H); 6.14-6.01(m, 2H); 3.8(s, 6H); 3.12-3.10(m, 3H); 2.63-2.62 (m, 1H); 2.18-2.13(m, 1H).
Embodiment 3
[0030] Preparation of compound 11:
[0031] Dissolve 44 grams (0.114mol) of compound 10 in 600ml of dichloromethane, add 19.15 grams (0.228mol) of sodium bicarbonate 19.15 grams (0.228mol) dissolved in 100ml of water, stir vigorously, and cool the reaction to 0 with an ice-water bath °C, add 41 g (0.171 mol) m-chloroperoxybenzoic acid in batches, and stir overnight at room temperature. Then slowly add an aqueous solution of 17.8 grams (0.171mol) of sodium bisulfite, continue to stir for 1 hour, carefully add 300ml of 10% potassium carbonate aqueous solution, separate the liquids, and wash the organic phase once with 300ml of 10% potassium carbonate aqueous solution, and then use Wash with saturated brine (300ml×3), and dry over anhydrous sodium sulfate. After filtering and evaporating the solvent to dryness, the obtained crude product was subjected to column chromatography (eluent: n-hexane:ethyl acetate=30:1), and 34 g of the product was obtained (yie...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com