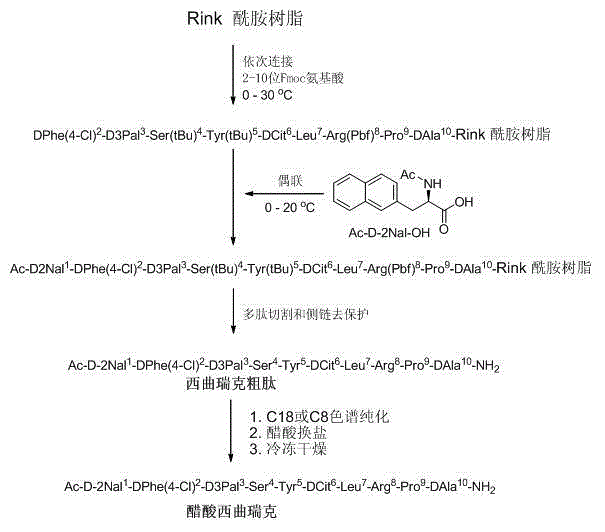
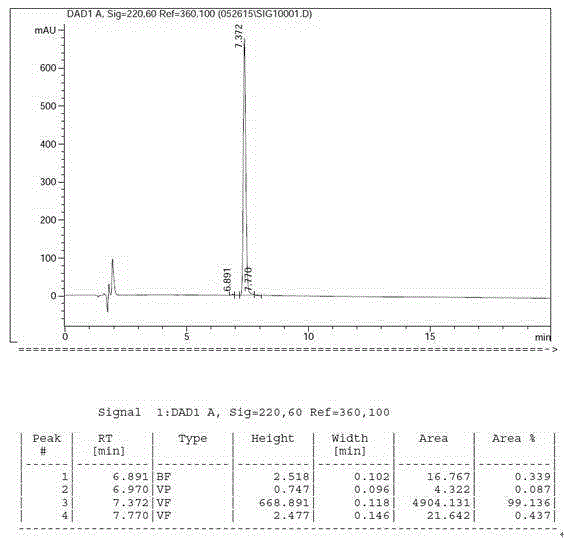
Preparation method of cetrorelix
A technology of cetrorelix and solid-phase synthesis, which is applied in the field of polypeptide drug preparation, and can solve problems such as product heavy metal pollution, process impurities, and environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Step 1. Fmoc Rink Amide resin (3.0 mmol, 5.1 g, degree of substitution 0.59 mmol / g) was placed in a reactor and swelled with N,N-dimethylformamide (100 ml) for 30 minutes. The suspension was filtered, and 20% piperidine / N,N-dimethylformamide (100 ml) was added to the resin and stirred for 30 min to remove the Fmoc protecting group. After Fmoc deprotection, the resin was washed thoroughly with N,N-dimethylformamide, ready for step 2 coupling.
[0047] Step 2. Dissolve 3.11 g of Fmoc-DAla-OH, 1.35 g of 1-hydroxybenzotriazole, and 1.58 mL of N,N-diisopropylcarbodiimide in N,N-dimethylformamide , to activate amino acids at 0 - 10 degrees Celsius for 20 minutes. After activation, the solution was added to the reactor containing the peptide resin, and then 2.2 mL of N-methylmorpholine was also added to the reactor of the resin. The coupling reaction is performed for 1 - 4 hours, or until the ninhydrin test is negative. The resin suspension was filtered and the peptide resi...
Embodiment 2
[0061] The nonapeptide resin was synthesized according to the method from step 1 to step 10 in Example 1, and then Ac-D-2Nal-OH was connected.
[0062] Add Ac-D-2Nal-OH (78 mg, 0.3 mmol) and o-(7-azabenzotriazol-1-yl)-1 to the nonapeptide resin (340 mg, 0.1 mmol) obtained in step 10, 1,3,3-Tetramethyluronium hexafluorophosphate (HATU) (114 mg, 0.3 mmol) in N,N-dimethylformamide. Then N,N-diisopropylethylamine (52 μl, 0.3 mmol) was added, and the coupling reaction was carried out at 0–5°C for 1–4 hours, or until the ninhydrin test was negative. The resin suspension was filtered and washed five times with N,N-dimethylformamide and twice with methanol. After drying under vacuum, the resin was then subjected to trifluoroacetic acid cleavage.
[0063] Trifluoroacetic acid cleavage: suspend the dried peptide resin in trifluoroacetic acid cutting solution, trifluoroacetic acid:water (95:5, 10 ml / g resin), and cut at room temperature for 2.5 hours. After filtration, the filtrate wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com