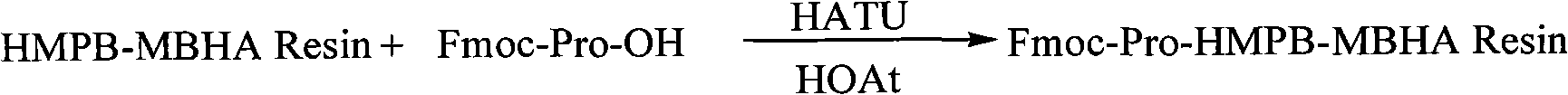Solid-phase preparation method for buserelin
A solid-phase preparation and solid-phase synthesis technology, which is applied in the preparation methods of peptides, chemical instruments and methods, organic chemistry, etc., can solve the problems of low application value, unfavorable industrial production, complicated operation, etc., and achieves easy post-processing, The effect of less environmental pollution and simple reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
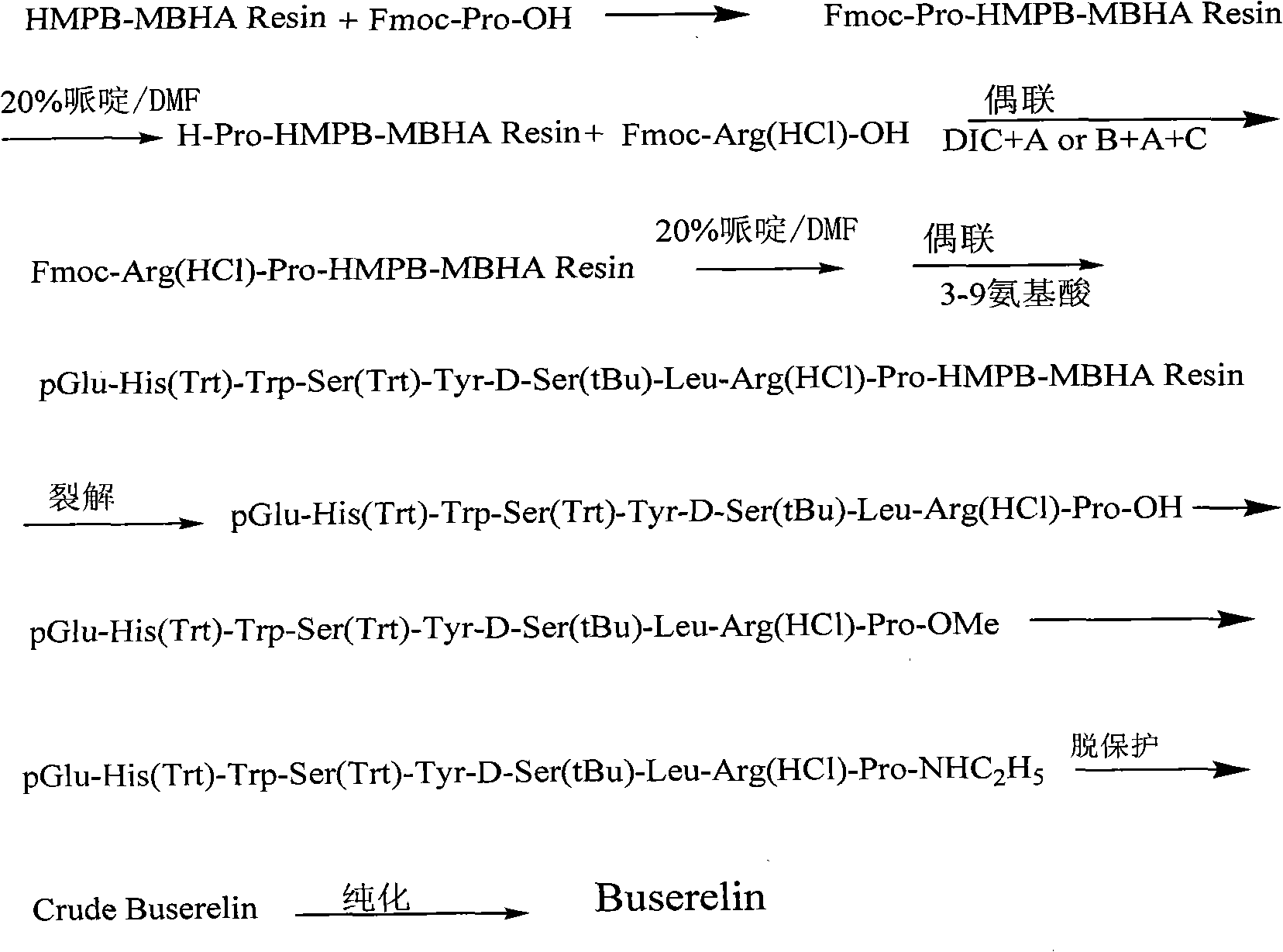
[0031] Embodiment 1: Preparation of Fmoc-Pro-HMPB-MBHA resin
[0032] 11.1g of HMPB-MBHA resin (Resin) with a substitution degree of 0.9mmol / g was added to the solid-phase reaction column, and after adding DCM to swell the resin for 30 minutes, 7.76g of Fmoc-Pro-OH, 8.30g of HATU, 2.98g of HOAt It was dissolved in DMF under ice bath, added to the above resin and reacted for 10 minutes, then added 2.4ml of TMP, and reacted at room temperature for 45 minutes. After washing with DMF for 3 times, washing with DCM for 3 times, and shrinking with methanol for 3 minutes, 5 minutes and 8 minutes respectively, the Fmoc-Pro-HMPB-MBHA resin was obtained by shrinking, and the detected substitution degree was 0.6mmol / g.
[0033] The route is as follows:
[0034]
Embodiment 2
[0035] Embodiment 2: Preparation of Buserelin-HMPB-MBHA resin
[0036] Weigh 10mmol Fmoc-Pro-HMPB-MBHA resin into the reactor, swell with DCM for 0.5 hours, then use 20% DBLK to remove the Fmoc protection twice for 10 minutes and 5 minutes respectively, and connect Fmoc-Arg (HCl )-OH. Dissolve 12.9g Fmoc-Arg(HCl)-OH, 4.9g HOBt, and 6.1ml DIC in DCM (a small amount of DMF can be added to aid dissolution), activate in an ice-water bath for 7 minutes, add to a solid-phase reactor, and react at room temperature for 1- 2 hours. The end point of the reaction was determined by the ninhydrin method. Repeat the above steps to sequentially complete the connection of the remaining amino acids to obtain Buserelin-HMPB-MBHA resin (Buserelin-HMPB-MBHA resin). Wherein the amount of raw materials: amino acid 3.0mmol, DIC 6.1ml, HOBt 4.9g.
Embodiment 3
[0037] Example 3: Preparation of pGlu-His(Trt)-Trp-Ser(Trt)-Tyr-D-Ser(tBu)-Leu-Arg(HCl)-Pro-OH
[0038] 1. Add 23.6g of Buserelin-HMPB-MBHA Resin into a 100ml round bottom flask.
[0039] 2. Prepare 200ml of lysis reagent, including 2ml of trifluoroacetic acid and 198ml of DCM, and pre-cool in the refrigerator for 30 minutes.
[0040] 3. Pour the cleavage reagent into the resin, ice bath while stirring, and blow nitrogen. After reacting for 30 minutes, the ice bath was removed, and the reaction was continued for 2 hours at room temperature. Filter the resin and collect the filtrate. The resin was washed with a small amount of DCM, and the filtrates were combined. The filtrate was slowly added to 800ml of glacial ether, and a white precipitate appeared. Centrifuge at 3000 rpm, wash with glacial ether for 5 times, and dry under reduced pressure to obtain 18.3 g of crude peptide, HPLC purity > 90%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com