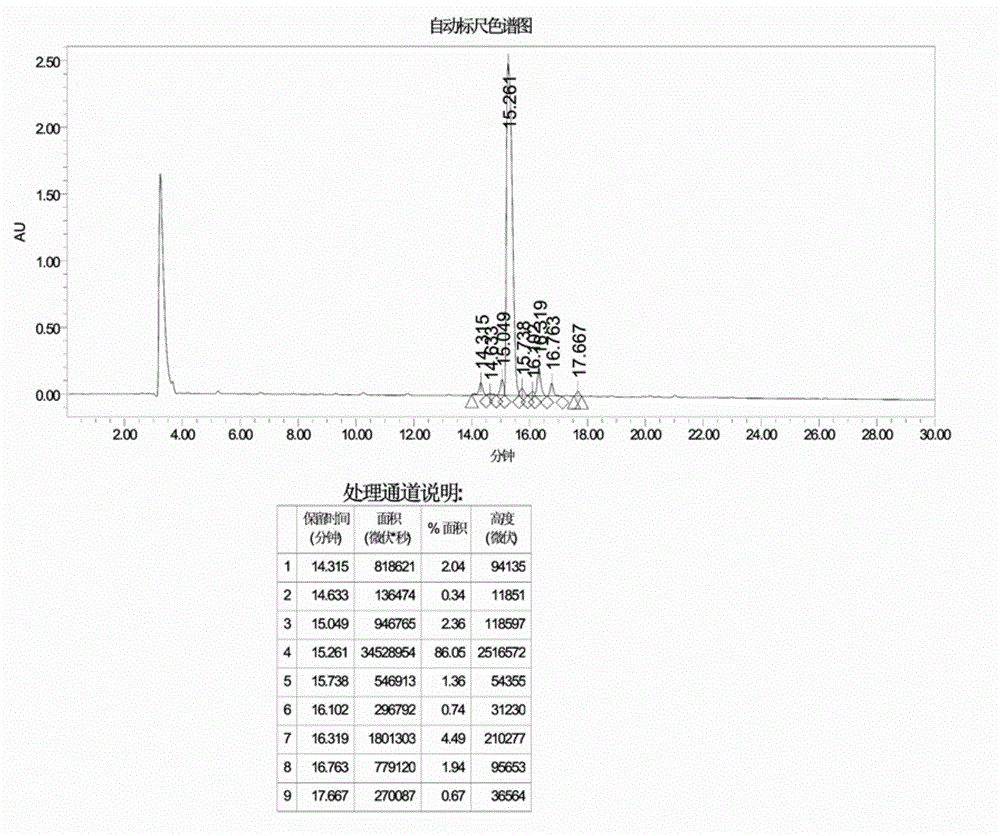
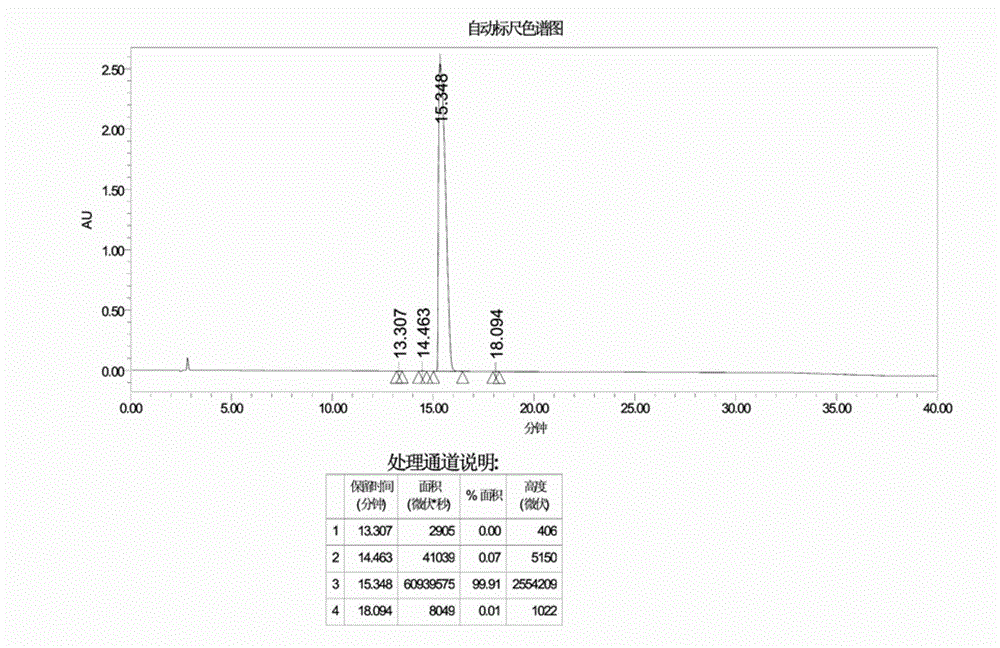
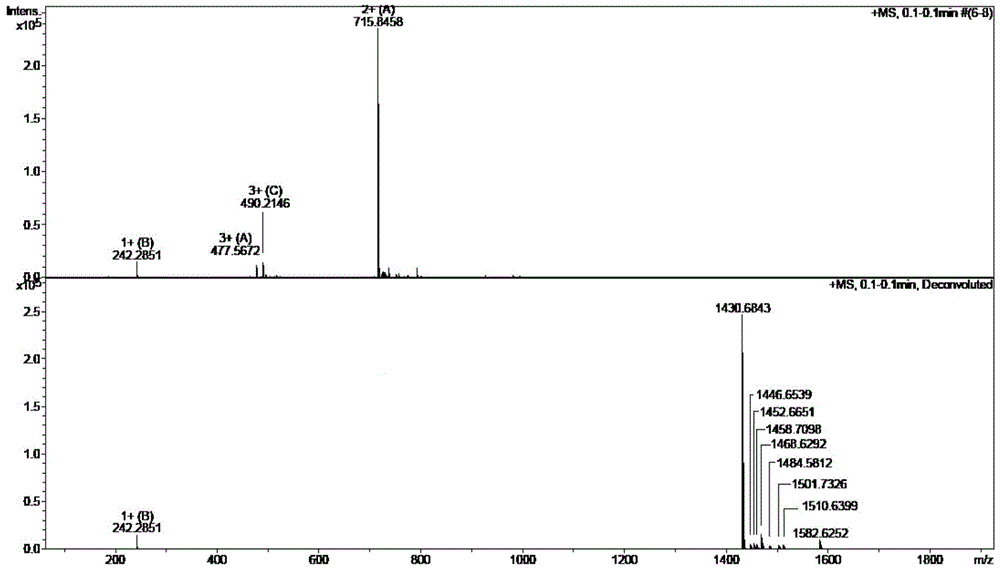
Preparation method of cetrorelix
A technology of cetrorelix and reagents, which is applied in the field of solid-phase synthesis of cetrorelix, can solve the problems of unfavorable large-scale industrial production of cetrorelix, low crude peptide yield, cumbersome process steps, etc., and achieve effective It is conducive to large-scale industrial production, has little impact on the human body and the environment, and has simple and stable processes.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] (1) Preparation of Fmoc-D-Ala-AM-resin
[0106] Weigh 1.62g (1.0mmol) Fmoc-Rink-Amide AM resin (substitution value 0.62mmol / g), place it in a jacketed 100mL polypeptide solid-phase synthesizer, add 20mL DMF to swell twice, each time for 1 hour . After the resin swelled completely, remove the DMF with a diaphragm pump, remove the Fmoc protecting group twice with 15 mL of 20% (v / v) Piperidine / DMF solution for 5 min and 15 min respectively, then wash with 20 mL of DMF for 5 times, and then remove the Fmoc protection group with a diaphragm pump. DMF was withdrawn. Dissolve 934.0mg (3.0mmol) Fmoc-D-Ala-OH and 405.3mg (3.0mmol) HOBt in 5mL DMF. After ultrasonic vibration and dissolution, ice bath for 10min, then add 560.0μL (3.6mmol) DIC, activate for 5min and mix The solution was added into a solid-phase reactor, stirred mechanically for 1 h, and the reaction temperature was controlled at 25° C. with constant temperature circulating water. The ninhydrin test was negative,...
Embodiment 2
[0122] (1) Preparation of Fmoc-D-Ala-AM-resin
[0123] Weigh 25.0g (15.5mmol) Fmoc-Rink-Amide AM resin (substitution value 0.62mmol / g), place it in a jacketed 500mL polypeptide solid-phase synthesizer, add 250mL DMF to swell twice, each time for 1 hour . After the resin swells completely, remove the DMF with a diaphragm pump, remove the Fmoc protecting group twice with 200mL 20% (v / v) Piperidine / DMF solution for 5min and 15min respectively, wash with 250mL DMF for 5 times, and then remove the Fmoc protecting group with a diaphragm pump. DMF was withdrawn. Dissolve 12.1g (38.8mmol) Fmoc-D-Ala-OH and 5.3g (38.8mmol) HOBt in 70mL DMF. After ultrasonic vibration and dissolution, ice bath for 10min, then add 7.2mL (46.5mmol) of DIC, activate for 5min The mixed solution was added into a solid-phase reactor, stirred mechanically for 1 h, and the reaction temperature was controlled at 25° C. with constant temperature circulating water. The ninhydrin test was negative, that is, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com