Preparation method for degarelix
A technology of degarelix and solid-phase synthesis, which is applied in the field of industrialized preparation of degarelix and degarelix acetate, and can solve problems such as potential safety hazards in transportation and production processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
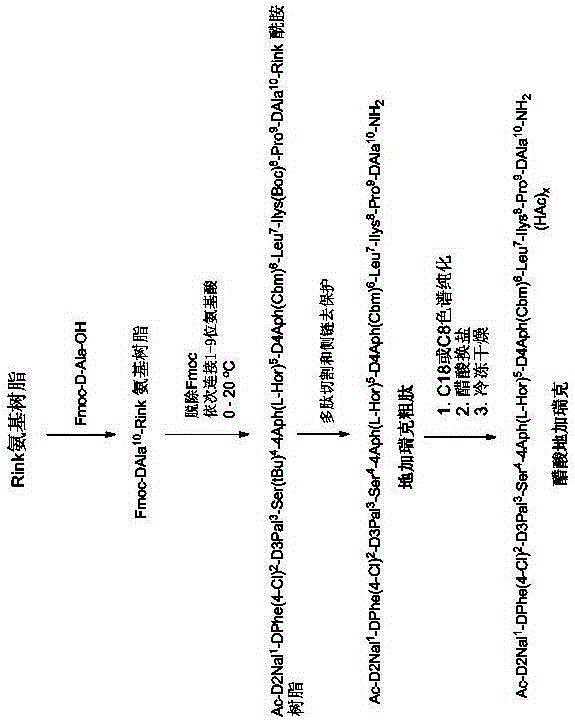
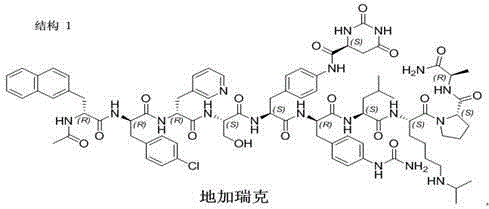
[0074] Step 1. FmocRinkAmide resin (800mmol, 2222g, degree of substitution 0.36mmol / g) was placed in a reactor and swelled with N,N-dimethylformamide (30L) for 30 minutes. The suspension was filtered, and 20% piperidine / N,N-dimethylformamide (30 L) was added to the resin and stirred for 30 min to remove the Fmoc protecting group. After Fmoc deprotection, the resin was washed thoroughly with N,N-dimethylformamide, ready for step 2 coupling.
[0075] Step 2. Dissolve 497.6g of Fmoc-DAla-OH, 227.4g of ethyl 2-oxime cyanoacetate (Oxyma) and 248mL of N,N-diisopropylcarbodiimide in N,N-dimethylformamide to make the amino acid Activate for 20 minutes at 0-10 degrees Celsius. After activation, the solution is added to the reactor containing the peptide resin, and the coupling reaction is carried out at 0-20 degrees Celsius for 1-4 hours, or until the ninhydrin test result is negative. The resin suspension was filtered and the peptide resin was washed with N,N-dimethylformamide. The...
Embodiment 2
[0089] Ac-D-2Nal-OH was connected by DIC / HOOBt coupling method.
[0090] Peptide Resin 1 was synthesized following steps 1 to 10 described in Example 1. The peptide resin 1 (17.2 g) obtained in step 10 above was stirred in a 20% piperidine / N,N-dimethylformamide solution for 30 minutes to remove the Fmoc protecting group. After Fmoc deprotection, the resin was washed thoroughly with N,N-dimethylformamide and was ready for coupling with Ac-D-2Nal-OH. Add 2.06g Ac-D-2Nal-OH and 1.3g HOOBt to the reactor containing the peptide resin, add an appropriate amount of N,N-dimethylformamide to dissolve the amino acid, and then add 1.24mL N,N-diisopropylcarbobis The imine is put into the reactor for the coupling reaction, and the coupling reaction is carried out at 0-20 degrees Celsius for 1-4 hours, or until the ninhydrin test result is negative. The resin suspension was filtered and washed five times with N,N-dimethylformamide and twice with methanol. After drying under vacuum, 19.85...
Embodiment 3
[0094] Ac-D-2Nal-OH was linked by DIC / HOBt coupling method.
[0095] Peptide Resin 1 was synthesized following steps 1 to 10 described in Example 1. The peptide resin 1 (0.88 g) obtained in step 10 above was stirred in a 20% piperidine / N,N-dimethylformamide solution for 30 minutes to remove the Fmoc protecting group. After Fmoc deprotection, the resin was washed thoroughly with N,N-dimethylformamide and was ready for coupling with Ac-D-2Nal-OH. 0.106 g Ac-D-2Nal-OH, 0.054 g HOBt and 0.062 mL N,N-diisopropylcarbodiimide were dissolved in N,N-dimethylformamide to activate the amino acid at 0-10 degrees Celsius for 60 minutes. After activation, the solution is added to the reactor containing the peptide resin, and the coupling reaction is carried out at 0-20 degrees Celsius for 1-4 hours, or until the ninhydrin test results are negative. The resin suspension was filtered and washed five times with N,N-dimethylformamide and twice with methanol. After drying under vacuum, 1.0 g ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com