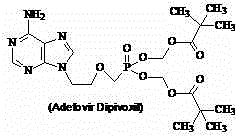
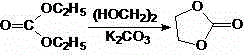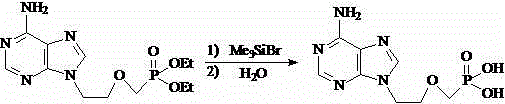Adefovir dipivoxil monohydrate and preparation method thereof
A technology of adefovir dipivoxil and monohydrate, which is applied in the field of drug synthesis, can solve the problems of cumbersome operation, difficult preparation of adefovir dipivoxil hydrate, and unsuitability for large-scale production, and achieve simple and convenient operation and clinical convenience Effects of application, good stability and water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The preparation of embodiment 1 ethylene carbonate (intermediate I)
[0025]
[0026] Install a mechanical stirrer, thermometer and reflux condenser in a dry 10 L reaction flask. Add 3 kg (48.39 mol) of ethylene glycol, 6.24 kg (52.88 mol) of diethyl carbonate and 16.3 g of anhydrous potassium carbonate of about 40% of the calculated amount in sequence, stir, and heat to reflux, react for 30 minutes, and then The remaining 24.0 g of anhydrous potassium carbonate was added. Continue the reflux reaction for 5 hours, recover ethanol, cool and crystallize, filter, and wash the filter cake with cold absolute ethanol, then air-dry to obtain ethylene carbonate as a colorless crystalline solid 2.23g, Mp: 36~38°C , yield 52.4%, Mp: 39-40°C, yield 51%-59.5%.
Embodiment 2
[0027] Example 2 Preparation of 9-hydroxyethyladenine (intermediate II)
[0028]
[0029] Install a mechanical stirrer, a reflux condenser and a thermometer in a 10L reaction flask. 3kg (22.22mol) of adenine, 2.14kg (24.32mol) of ethylene carbonate, 20g (0.5mol) of sodium hydroxide and 7L of DMF were sequentially added thereto, stirred, and heated to reflux for 4 hours. After the reaction is completed, cool to room temperature, filter, wash the filter cake with DMF, and dry it in vacuum at 70° C. for 6 hours. The obtained 9-hydroxyethyladenine was 3.29kg of off-white solid powder, Mp: 225-227°C (decomposition), and the yield was 82.7%.
Embodiment 3
[0030] Example 3 Preparation of 9-[2-(diethoxyphosphorylmethoxy)ethyl]adenine (intermediate III)
[0031]
[0032] Install a mechanical stirrer, a dropping funnel and a thermometer in a 50 L dry reaction flask. Add 2kg (11.17mol) of intermediate II and 12L of DMF to it in sequence, stir, and cool to below 0°C, add 1.1kg (45.83mol) of 60% sodium hydride in batches to control the feeding speed, so that the reaction temperature is between 0 and 5°C between. After the addition is completed, continue to stir and react at 5°C for 1 hour, then add dropwise a solution containing 4.7kg (14.60mol) of diethyl toluenesulfonyloxymethyl phosphate and DMF8.5L, and control the rate of addition so that the reaction temperature is between Between 0 and 5°C, after the addition is complete, continue to stir and react at 5°C for 5 hours. After the reaction was completed, add glacial acetic acid to neutralize and adjust the pH value to 5-6, distill under reduced pressure and recover DMF, disso...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com