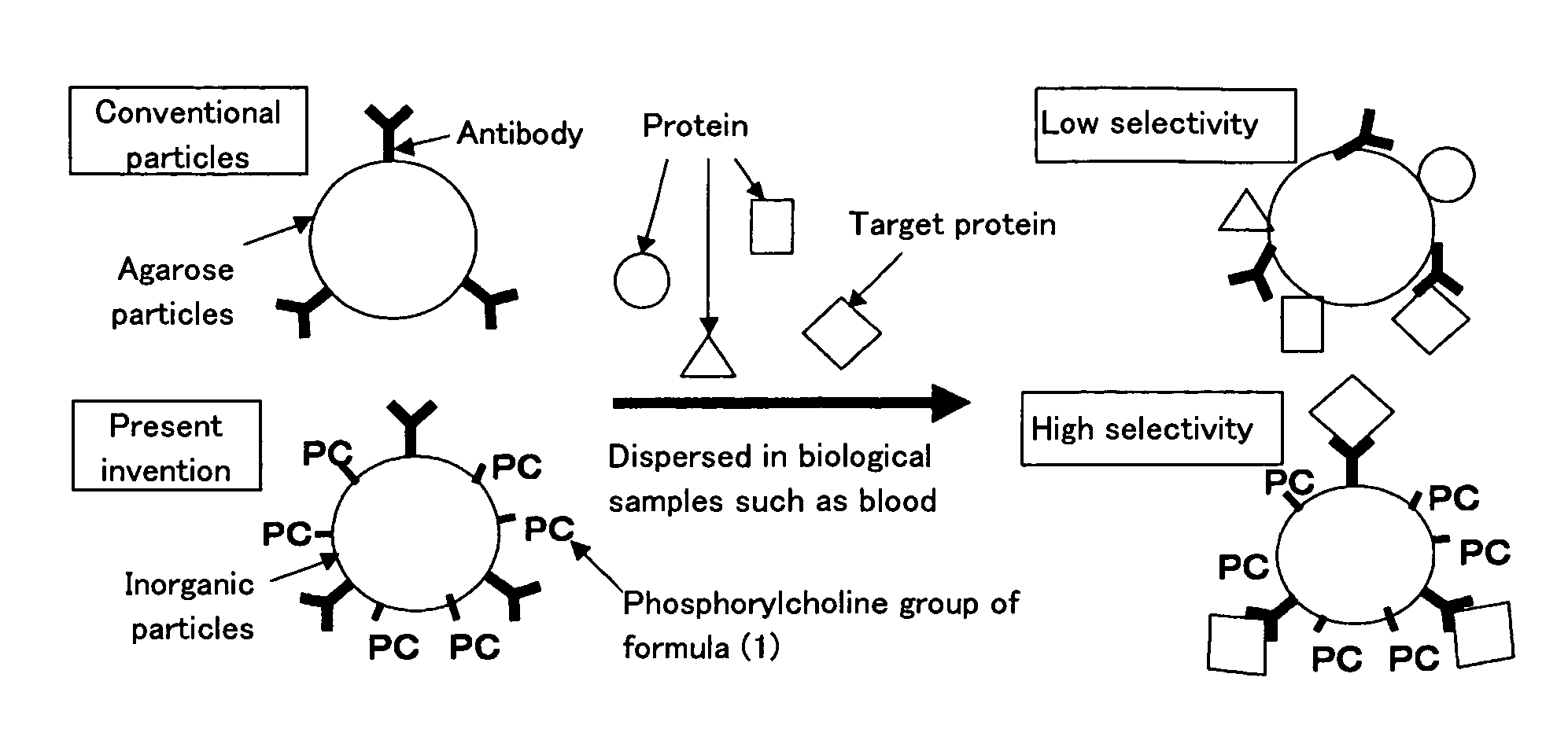
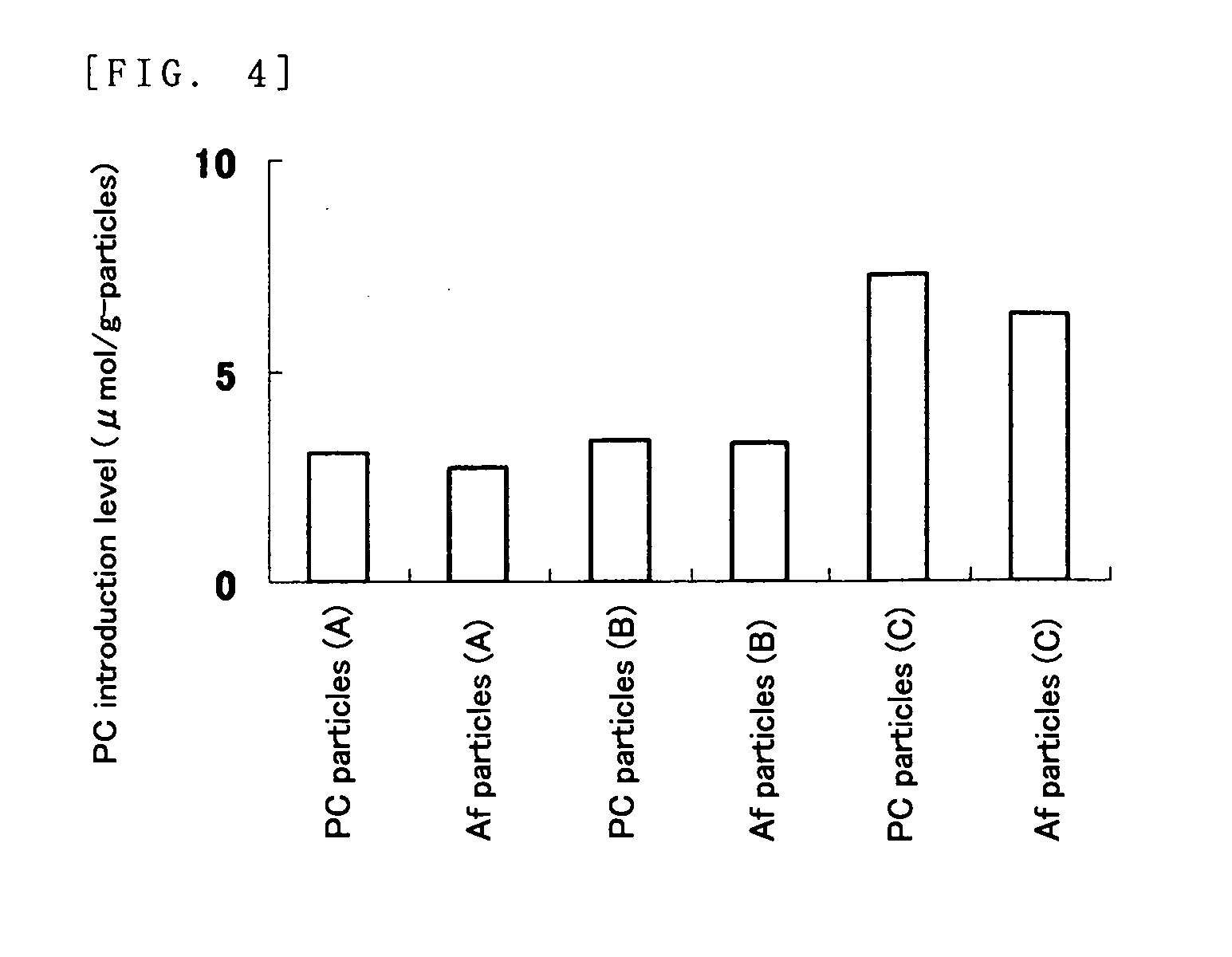
Affinity particle and affinity separation method
a technology of affinity separation and affinity particles, which is applied in the field of affinity particles and affinity separation methods, can solve the problems of large quantity of sample, poor purification efficiency, and time-consuming purification, and achieves superior dispersion properties, high separation selectivity, and suppression of adsorption of other substances.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
synthesis example 1
“An aldehyde Chemical Compound Containing Phosphorylcholine Groups”
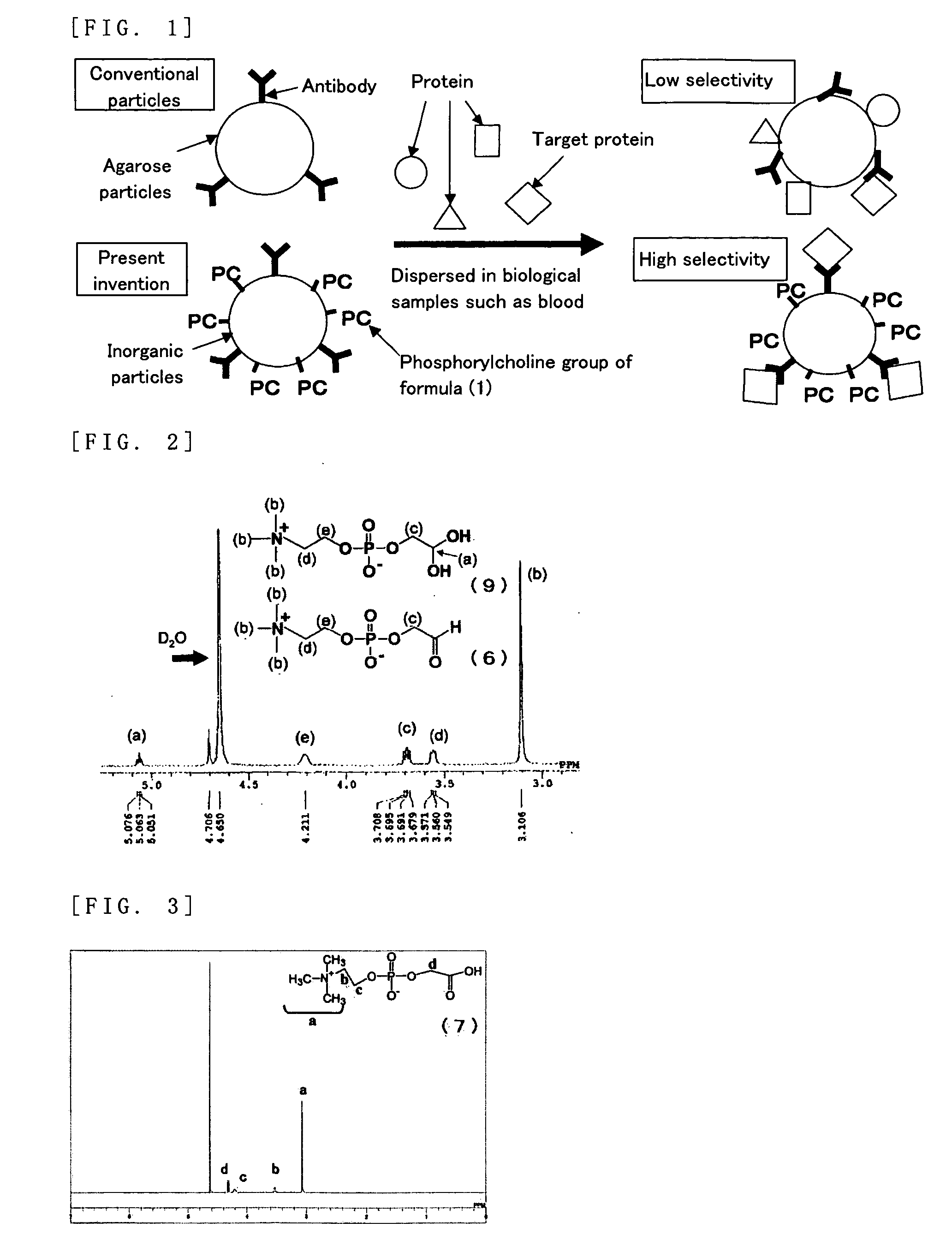
[0135] 1-alpha-glycerophosphorylcholine (6.29 g) was dissolved in 210 ml of distilled water and cooled in an ice water bath. Sodium periodate (10.23 g) was added, followed by five hours of stirring. The reaction fluid was concentrated under reduced pressure and dried under reduced pressure; methanol was then used to extract the target substance. The structure is shown in the following chemical formula (6).
[0136] A 1H NMR spectrum of the compound of formula (6) is shown in FIG. 2. Since the compound of formula (6) is in equilibrium with formula (9) in water, the actual spectrum reflects both formula (6) and formula (9).
synthesis example 2
“A Carboxylic Acid Chemical Compound Containing Phosphorylcholine Groups”
[0137] 5 g of glycerophosphorylcholine, 17 g of sodium periodate, 81 mg of ruthenium trichloride n-hydrate, 70 g of ion-exchanged water and 30 g of acetonitrile were put into a 200 ml flask. After stirring for two hours at room temperature, filtering was carried out and the solvent was removed from the filtrate. Methanol was used to extract the target compound from the obtained solid product; methanol was then removed to obtain the target compound (7).
[0138] A 1H NMR spectrum of the compound of formula (7) is shown in FIG. 3.
synthesis example 3
“Compound of Formula (10)”
[0139] 9.0 g of the compound of Synthesis example 1 was dissolved in 55 mL of dehydrated methanol, and the air inside the vessel was replaced by dry nitrogen. Next, 2.84 g of 3-aminopropyltrimethoxysilane was added to the methanol solution of chemical compound 1. This mixed solution was stirred overnight at room temperature and cooled with ice; 1.39 g of sodium cyanohydroborate was then added and the temperature was returned back to room temperature, followed by 5 hours of stirring. During this time dry nitrogen was continued to be fed through the reaction vessel. After filtering the precipitation, a methanol solution of the target substance, i.e. the compound of the following formula (10), was obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com