CYP101 enzyme recombinant vector, construction method thereof and CYP101 enzyme high-efficiency expression and purification method
A recombinant vector and high-efficiency expression technology, which is applied in the field of CYP101 enzyme recombinant vector and construction, high-efficiency expression and purification of CYP101 enzyme, can solve the problems of reduced enzyme activity, low protein purity, and difficulty in ensuring enzyme activity, and achieve high expression level and protein structure. small effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
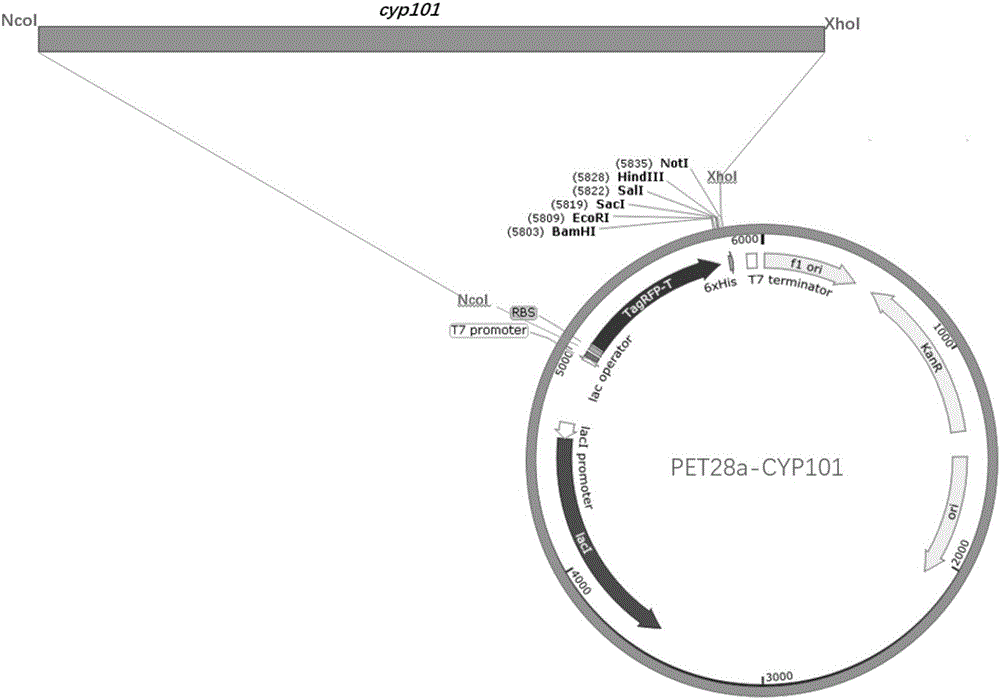
[0079] Example 1: Vector construction of PET28a-CYP101
[0080] 1. Template: extract the whole genome of Pseudomonas putida and use it as a template for PCR amplification reaction. The source of this example is 700412 TM , this product is a mature product and can be purchased directly.
[0081] 2. PCR amplification: using the whole genome of Pseudomonas putida as a template, the amplification product is obtained through PCR amplification reaction;
[0082] Among them, the designed upstream and downstream primer sequences are respectively:
[0083] Upstream primer F: CCGCCATGGCGATGGTTCCCAGCGACCTGTATC
[0084] Downstream primer R: GCGGCGGCCGCCTCGCTGTCAAACTTAATAGPCR
[0085] The PCR reaction system includes: 10× amplification buffer (containing Mg 2+ ) 5 μl, 2.5M dNTP mix 2 μl, 10 μM primer 1 μl, template DNA 2 μl, Taq DNA polymerase 1 μl, add ddH2O to 50 μl.
[0086] After mixing the components of the reaction system, put them into the PCR instrument, and set the cycle c...
Embodiment 2
[0094] Example 2: Massive Induced Expression of CYP101
[0095] 1. Transformation: Transform 2 μl of the recombinant plasmid PET28a-CYP101 into Escherichia coli BL21-plysS competent bacteria, and streak it on the Kanabio LB solid medium plate, and place it in a constant temperature incubator at 37°C for 12-16 hours.
[0096] 2. Seed culture: Pick the monoclonal colony and culture it in a 20ml LB liquid medium flask with shaking at 37°C, shake the bacteria overnight at 250rpm.
[0097] 3. Expanded culture: Transfer the bacterial solution obtained in step 2 to 1LTB medium containing Cannabidiol at an inoculation ratio of 1:500, add 250 μl trace elements (trace elements, known products purchased directly), 37°C , 200rpm shaking culture.
[0098] 4. Induced expression: Cultivate the Erlenmeyer flask under the above conditions for nearly 3 hours until OD600=0.5-0.7, take 1ml of bacterial liquid as a negative control, and add IPTG (isopropyl-β- D thiogalactoside, Amresco 367-93-1)...
Embodiment 3
[0100] Embodiment 3: Purification of CYP101 protein
[0101] The whole process of purification of CYP101 protein was performed on ice.
[0102] A. Preparation of cell supernatant
[0103] a1. Bacteria resuspension: the bacteria collected in Example 2 were resuspended with cold buffer A (50 mM potassium phosphate, 50 mM potassium chloride, pH 7.4) (slowly blown on ice to homogenize).
[0104] a2. Lysozyme treatment: add lysozyme at a final concentration of 2 μg / ml, add PMSF protease inhibitor at a ratio of 1:200, and let stand on ice for 30 minutes.
[0105] a3. Ultrasonic crushing: Ultrasonic crushing, ultrasonic for 3 seconds, stop for 7 seconds, cycle mode, until the bacteria are clarified.
[0106] a4. High-speed centrifugation: 16000rpm, 15 minutes, centrifuge the broken liquid for 15 minutes at 4°C, collect the supernatant, repeat the centrifugation once, and collect the supernatant;
[0107] a5. Membrane filtration: The above supernatant must be filtered with a 0.45 μ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com