Technique for purifying recombined human interferon alpha 1b
A technology of recombinant human interferon and interferon α, which is applied in the separation and purification of protein and the separation and purification process of recombinant human interferon α1b, which can solve the problems of low production efficiency, reduced total yield, complex process, etc., and achieve production cost reduction , The effect of improving production efficiency and shortening the process cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Expanded bed adsorption gel selection and chromatography parameter optimization
[0039] The operation of EBA requires special chromatography columns to produce a stable expanded bed. In the initial stage of process development, experiments were first carried out in the form of packed beds. The reason for using a packed bed for gel selection and parameter optimization is that although the adsorption effect of the expanded bed is better, the conditions required for gel adsorption in the two methods are consistent and have no effect on the selection of gels and chromatographic parameters. And it can save the investment of small-scale expansion bed system. With a packed bed, since particulate impurities cannot be removed, the sample needs to be centrifuged first to obtain clarified material.
[0040] the gel
Experiment number
Column
mm*cm
buffer system
Loading amount
Elution point
collect
type
pH
Concentr...
Embodiment 2
[0044] Example 2: Exploration of interferon purification process using STREAMLINE DEAE packed bed column
[0045] On the laboratory scale, use XK16×10 column in the form of packed bed, choose STREAMLINE DEAE and Tris buffer solution with pH 7.4, and use the same batch of bacterial homogenate as the old process to produce pure interferon along the new process route. In the comparison between the new and the old process, the data of the old process is taken from the actual production.
[0046] Experimental Materials:
[0047] 1. Equipment: chromatography column: XK16×10, gel: STREAMLINE DEAE, chromatography system: Pharmacia GP-250 gradient controller
[0048] 2. Buffer: Equilibrium: pH7.4 50mM Tris-HCl, eluent: 0-0.5MNaCl pH7.450mM Tris-HCl Experimental procedure:
[0049] 1. Packing: Pack the column in the equilibrium buffer system at a flow rate of 20ml / min, and the volume of the column is 20ml
[0050] 2. Equilibrium: use 10ml / min flow rate, equilibrate the column bed wit...
Embodiment 3
[0058] Example 3: Establishment of expanded bed adsorption manual purification system
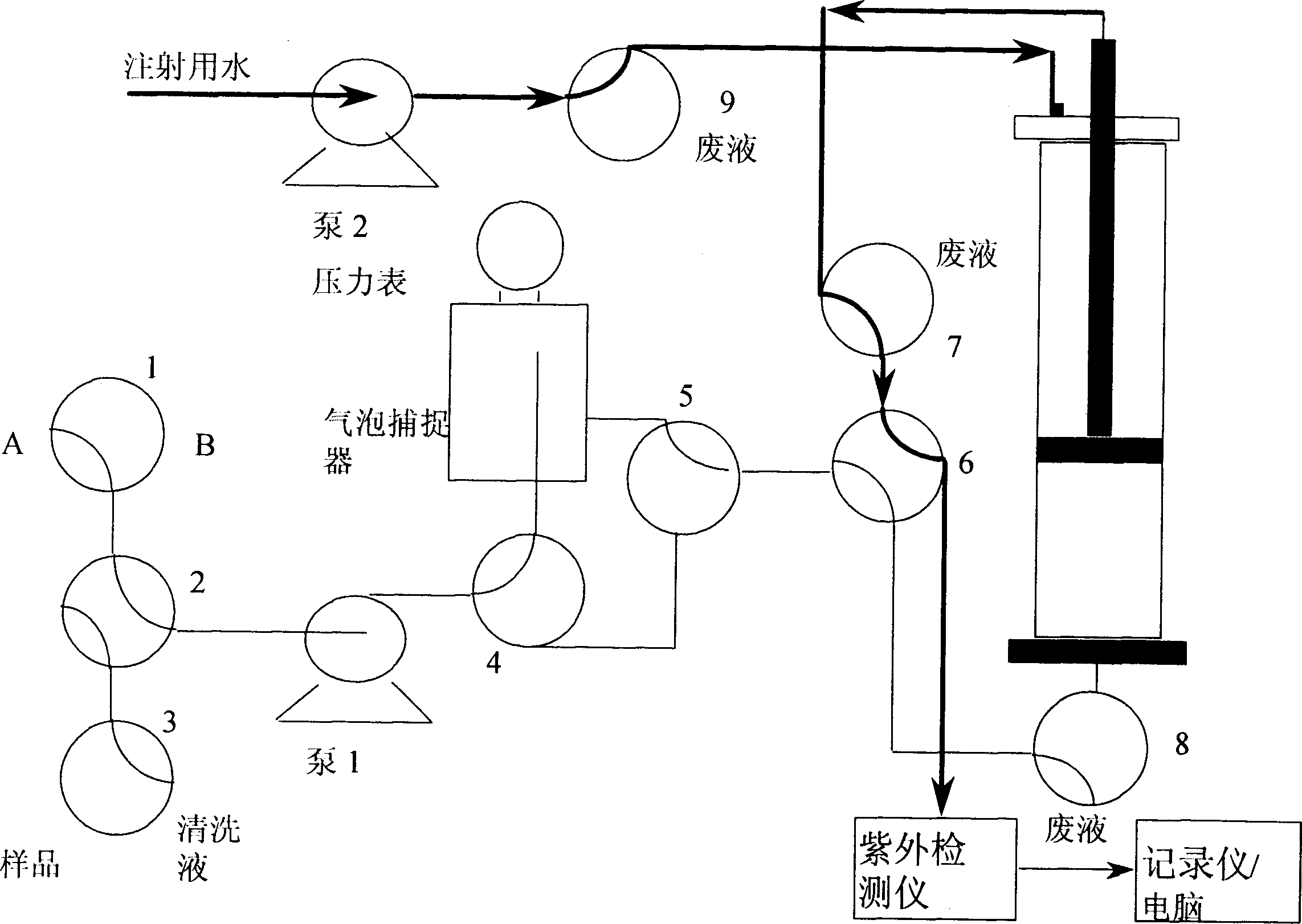
[0059] In the process of scaling up from the experimental scale to the production scale, it is faced with the problem of establishing an expanded bed adsorption purification system. Based on the consideration of reducing investment, we worked with the chromatography column supplier to establish a manual purification system for expanded bed adsorption.
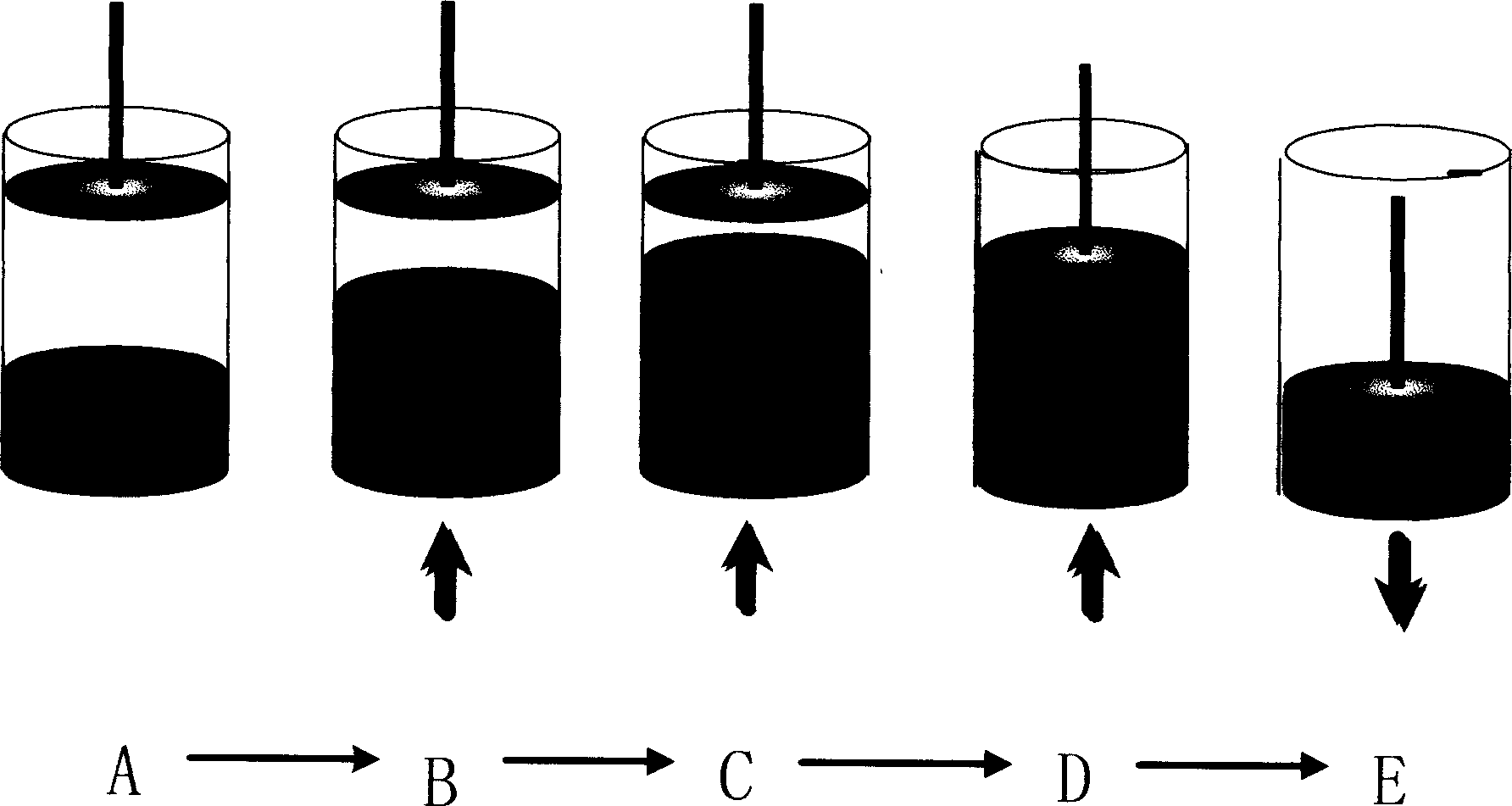
[0060] According to the principle diagram of the expanded bed adsorption operation in Figure 2, the steps of the expanded bed adsorption include equilibrium expansion, sample loading, column cleaning, sedimentation and elution, and column regeneration. In order to realize the two functions of expansion and chromatography at the same time, a double pump system must be used. One pump is responsible for the expansion and settling of the column bed, and the other pump is responsible for equilibration, sample loading, elution, and regeneration. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com