Alkyl-substituted ethyl acetate-based guanidine ionic liquid as well as preparation and application thereof
The technology of ethyl acetate-based guanidine and ethyl acetate-based tetramethyl guanidine is applied to liquid catalysts and application fields, and can solve the problems of complicated ionic liquid synthesis process, environmental pollution and high preparation cost, and achieves simple post-processing and avoidance of The effect of organic solvent, simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
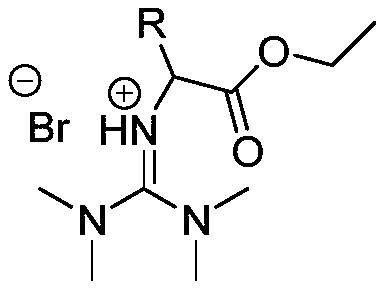
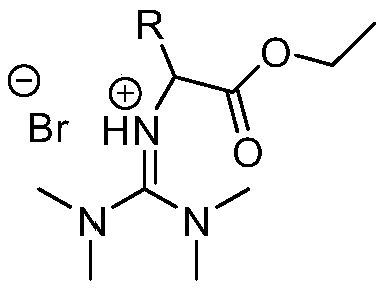
[0019] At a temperature of 60°C, dissolve 2.53g (22mmol) of tetramethylguanidine in 15mL of acetonitrile and mix it to form A solution, dissolve 3.34g (20mmol) of ethyl bromoacetate in acetonitrile to prepare B solution, and then dissolve B solution with 1 drop Slowly add it dropwise at a speed of 1 second, stir and mix in the A solution, stir the reaction system at a temperature of 60°C for 24 hours to carry out the ionization reaction of the following reaction structure formula:
[0020]
[0021] After the reaction finishes, acetonitrile is spin-dried on a rotary evaporator, then washed three times with ethyl acetate 25mL / time, and vacuum-dried at room temperature to obtain a light yellow product that is bromo-ethyl acetate-based tetramethylguanidine ionic liquid, and its yield is 86%.
Embodiment 2
[0023] At a temperature of 60°C, dissolve 2.53g (22mmol) of tetramethylguanidine in 15mL of acetonitrile and mix to form solution A, dissolve 3.62g (20mmol) of ethyl 2-bromopropionate in acetonitrile to form solution C, and then dissolve solution C Slowly add it dropwise at a rate of 1 drop / second, stir and mix in the A solution, and stir the reaction system at 60°C for 24 hours to carry out the ionization reaction of the following reaction structure formula:
[0024]
[0025] After the reaction was finished, the acetonitrile was spin-dried on a rotary evaporator, washed three times with ethyl acetate 25mL / time, and vacuum-dried at room temperature to obtain a light yellow product that was bromo-methylacetate-based tetramethylguanidine ionic liquid, which produced The rate is 84%.
Embodiment 3
[0027] At a temperature of 60°C, 2.53g (22mmol) of tetramethylguanidine was dissolved in 15mL of acetonitrile and mixed to form A solution, 5.02g (20mmol) of ethyl 2-bromooctanoate was dissolved in acetonitrile to prepare D solution, and then D solution was prepared with Slowly add dropwise at a rate of 1 drop / second, stir and mix in solution A, and stir the reaction system at 60°C for 24 hours to carry out the ionization reaction of the following reaction structure formula:
[0028]
[0029] After the reaction was finished, the acetonitrile was spin-dried on a rotary evaporator, washed three times with 25 mL / time of ethyl acetate, and vacuum-dried at room temperature to obtain a light yellow product that was bromo-n-hexyl ethyl acetate tetramethylguanidine ionic liquid, which produced The rate is 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com