1, 2, 4-triazole compound and preparation method thereof
A technology of hydrazine compounds and compounds, applied in the field of organic intermediates, can solve problems such as danger and explosive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0055] The present invention provides a preparation method of 1,2,4-triazole compounds described in the above scheme, comprising the following steps:
[0056] Mix hydrazine compounds, isothiocyanate compounds, tetramethylguanidine, photocatalysts and polar organic solvents to obtain a mixed solution; the mixed solution undergoes a cyclization reaction under light and a temperature of 50-80°C, Obtain the 1,2,4-triazole compound;
[0057] The structural formula of the hydrazine compound is R 1 -NHNH 2 ; The structural formula of the isothiocyanate compound is R 2 N=C=S; the photocatalyst is an organic dye and / or a metal complex.
[0058] The invention mixes hydrazine compound, isothiocyanate compound, tetramethylguanidine, photocatalyst and polar organic solvent to obtain mixed solution. In the present invention, the structural formula of the hydrazine compound is R 1 -NHNH 2 , R in the structural formula of hydrazine compounds 1 and R in the structure shown in formula I ...
Embodiment 1
[0069] A 1,2,4-triazole compound having a structure shown in formula I-(1):
[0070]
[0071] Preparation method: add 0.1mmol phenylhydrazine, 0.1mmol phenyl isothiocyanate, 0.15mmol tetramethylguanidine, and 1L acetonitrile to the reaction tube and mix, then add 0.001mmol rose bengal to it, and place the resulting mixture at 465nm Under the irradiation of light source, the reaction was stirred at 60°C for 24 hours. After the reaction, it was separated and purified by column chromatography. The volume ratio of petroleum ether and ethyl acetate in the column chromatography eluent was 5:1, and the purified target product was obtained. Yield 70%, purity 99.9%.
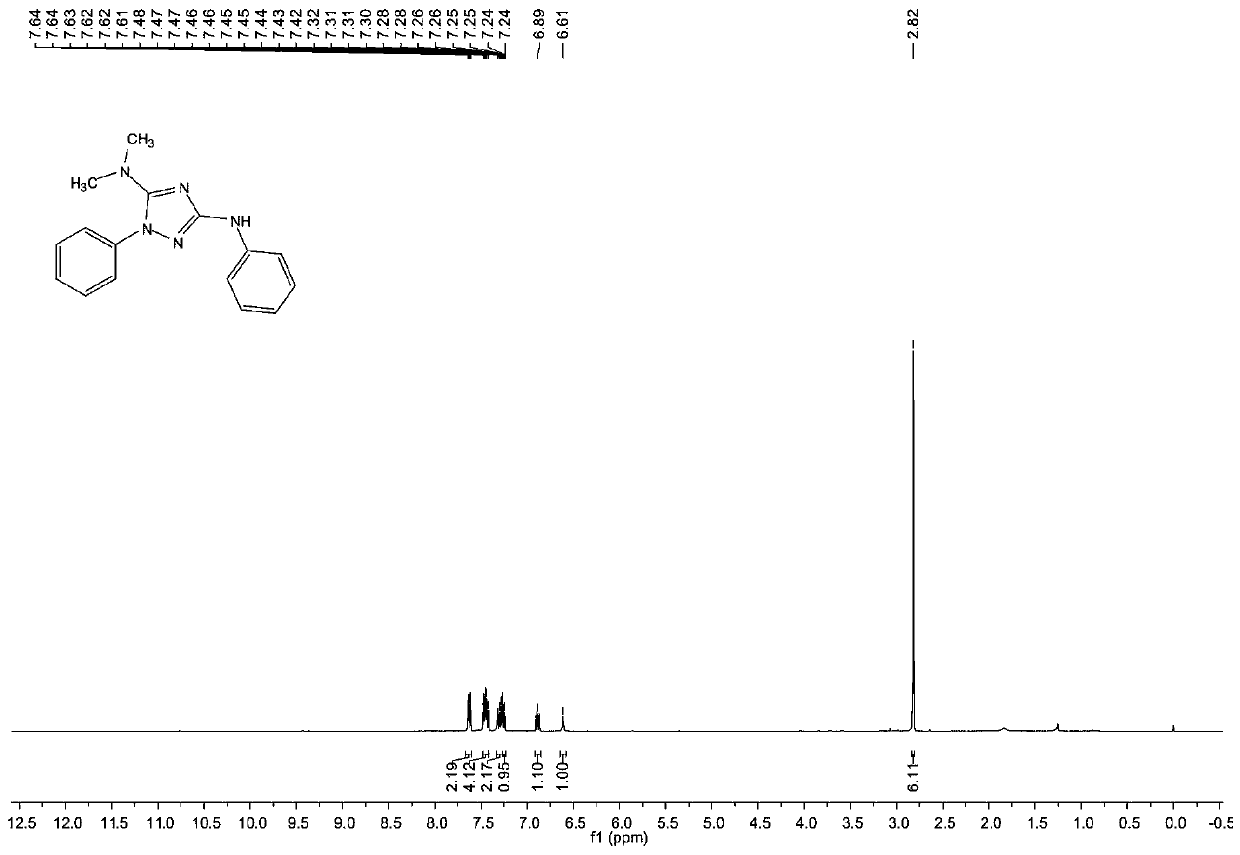
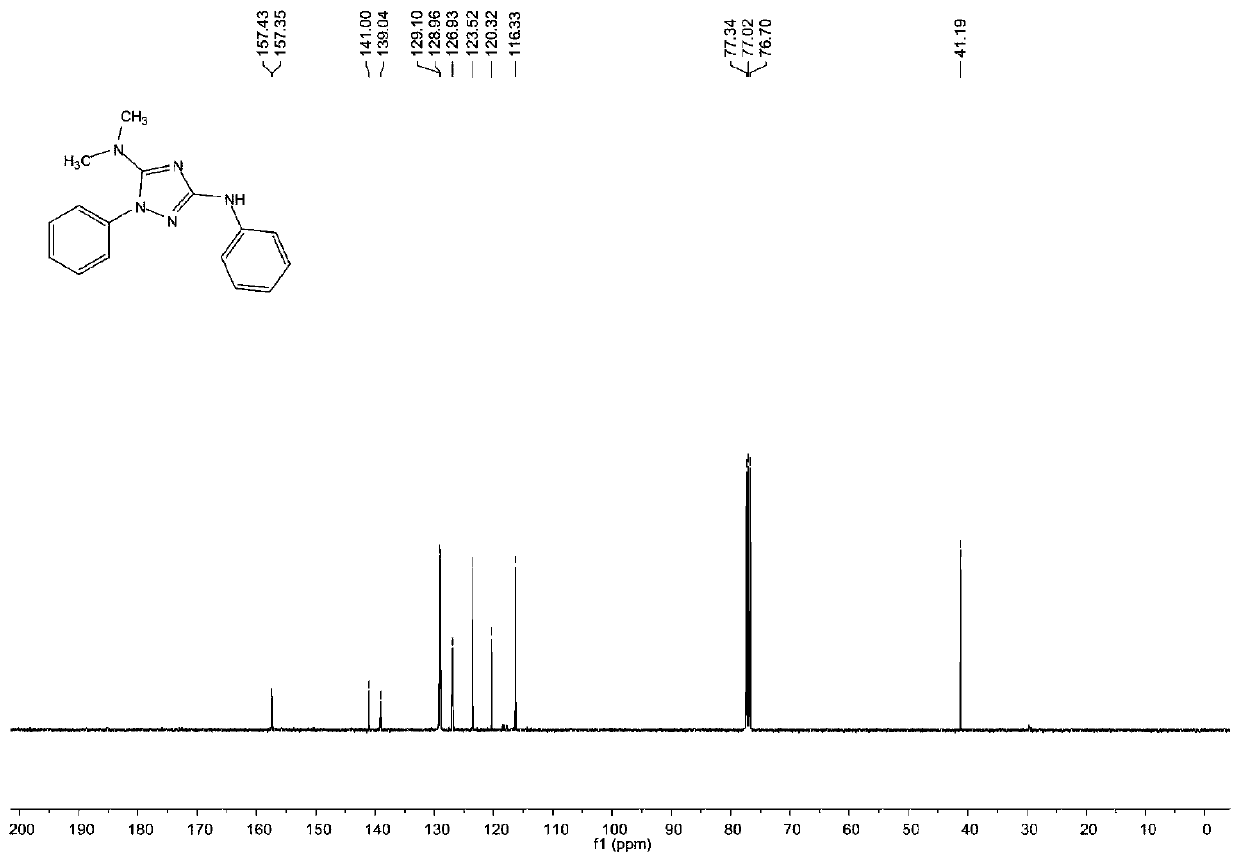
[0072] The structure of the obtained product is characterized, and the proton nuclear magnetic resonance spectrum and the carbon nuclear magnetic resonance spectrum are respectively as follows figure 1 with figure 2 As shown, the structural characterization data are as follows:
[0073] 1 HNMR (400MHz, CDCl 3 ,pp...
Embodiment 2
[0078] A 1,2,4-triazole compound having a structure shown in formula I-(2):
[0079]
[0080] Preparation method: Add 0.1mmol 4-chlorophenylhydrazine, 0.1mmol phenyl isothiocyanate, 0.15mmol tetramethylguanidine, and 1mL acetonitrile into a reaction tube and mix them, then add 0.001mmol Rose Bengal, and mix the resulting mixture Placed under the irradiation of a 465nm light source, stirred and reacted at 60°C for 12 hours, separated and purified by column chromatography after the reaction, the volume ratio of petroleum ether and ethyl acetate in the column chromatography eluent was 5:1, and the purified target was obtained The product has a yield of 34% and a purity of 99.9%.
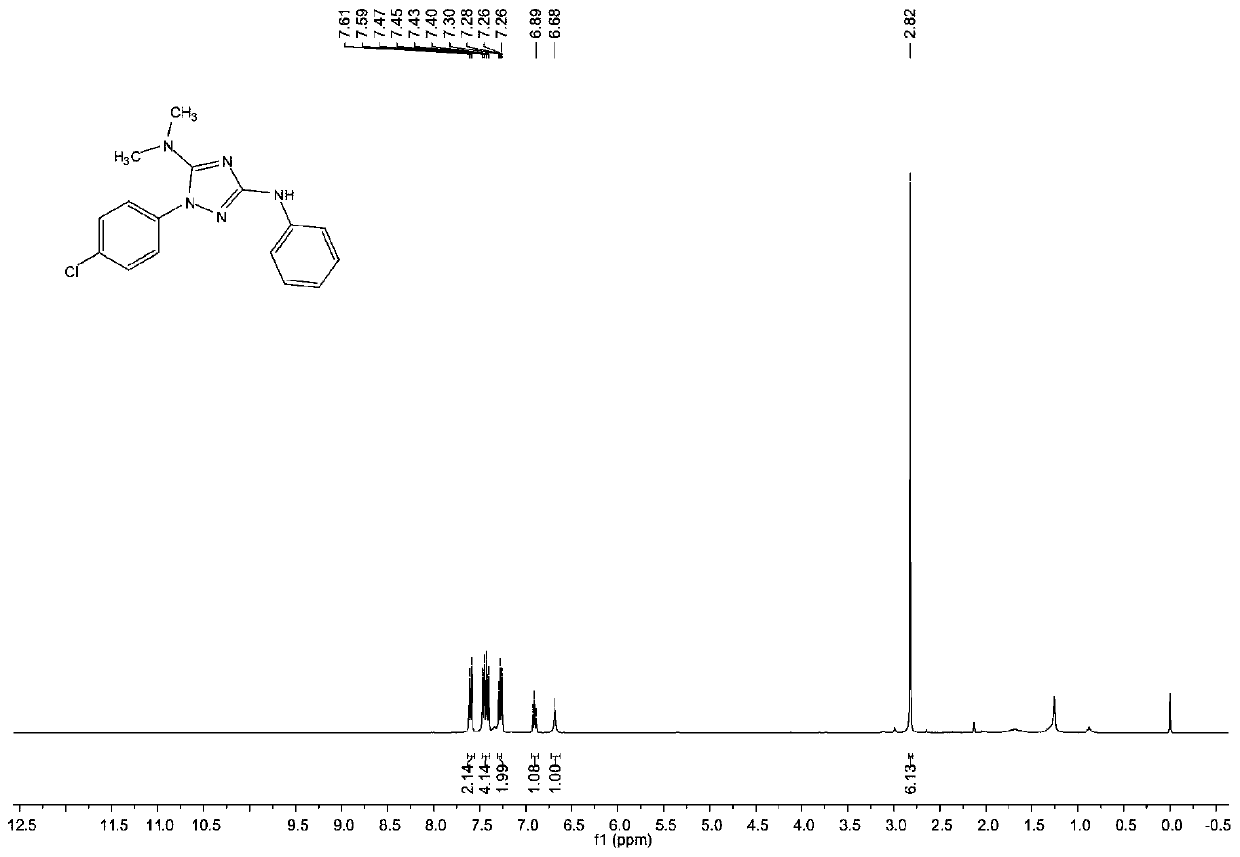
[0081] The structure of the obtained product is characterized, and the proton nuclear magnetic resonance spectrum and the carbon nuclear magnetic resonance spectrum are respectively as follows image 3 with Figure 4 As shown, the structural characterization data are as follows:
[0082] 1 HNMR (...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Light source wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com