5-alkyl guanidine ionic liquid, and preparation and application thereof
A technology of ionic liquid and pentaalkylguanidine, which is applied in the field of liquid catalyst and application, can solve the problems of complex ionic liquid synthesis process, polluting environment, preservation, and high production cost, and achieve good organic solubility, warm reaction conditions, and recyclable use Effects in simple steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
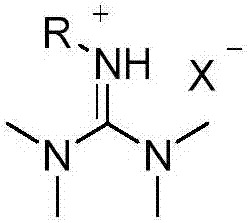
[0019] Under the protection of argon at 0°C, 2.10g (20mmol) of tetramethylguanidine was dissolved in 10ml of acetonitrile and mixed to form A solution, 2.40g (22mmol) of bromoethane was dissolved in 10ml of acetonitrile to form B solution, then B The solution was slowly added dropwise to solution A at a rate of 1 drop / second, stirred and mixed, and the reaction system was stirred at room temperature for 24 hours to carry out ionization reaction. After washing three times with 25 ml / time, and vacuum drying at room temperature, the white crystal product was bromo-N,N,N',N'-tetramethyl-N-ethylguanidine ionic liquid, and the yield was 88%.
Embodiment 2
[0021] Weigh 2.14g (10mmol) of the above-prepared bromine-N,N,N',N'-tetramethyl-N-ethylguanidine ionic liquid and dissolve it in 5ml of ethanol to prepare a C solution, weigh 1.90g (5mmol) of three The hydrated lead acetate was dissolved in 5 ml of ethanol to prepare the D solution, the C solution was mixed with the D solution at room temperature, and the reaction solution was filtered after stirring for 2 hours. After removing ethanol, the transparent and viscous product was N,N,N',N'-tetramethyl-N"-ethylguanidine acetate ionic liquid, and the yield was 93%.
Embodiment 3
[0023] Under the protection of argon at 0°C, 2.10g (20mmol) of tetramethylguanidine was dissolved in 10ml of acetonitrile and mixed to form E solution, 2.84g (20mmol) of methyl iodide was dissolved in 8ml of acetonitrile to form F solution, and then F solution was The speed of 1 drop / second was slowly added dropwise to the E solution, stirring and mixing, and the reaction system was stirred at room temperature for 12 hours to carry out ionization reaction. After washing three times, and drying under vacuum at room temperature, the white crystal product was obtained as iodine-N,N,N',N',N"-pentamethylguanidine ionic liquid, and the yield was 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com