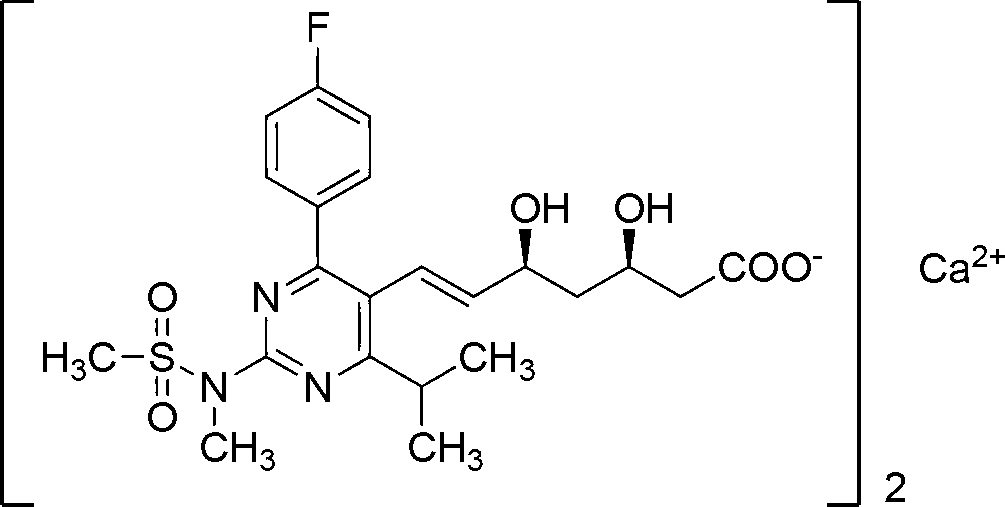
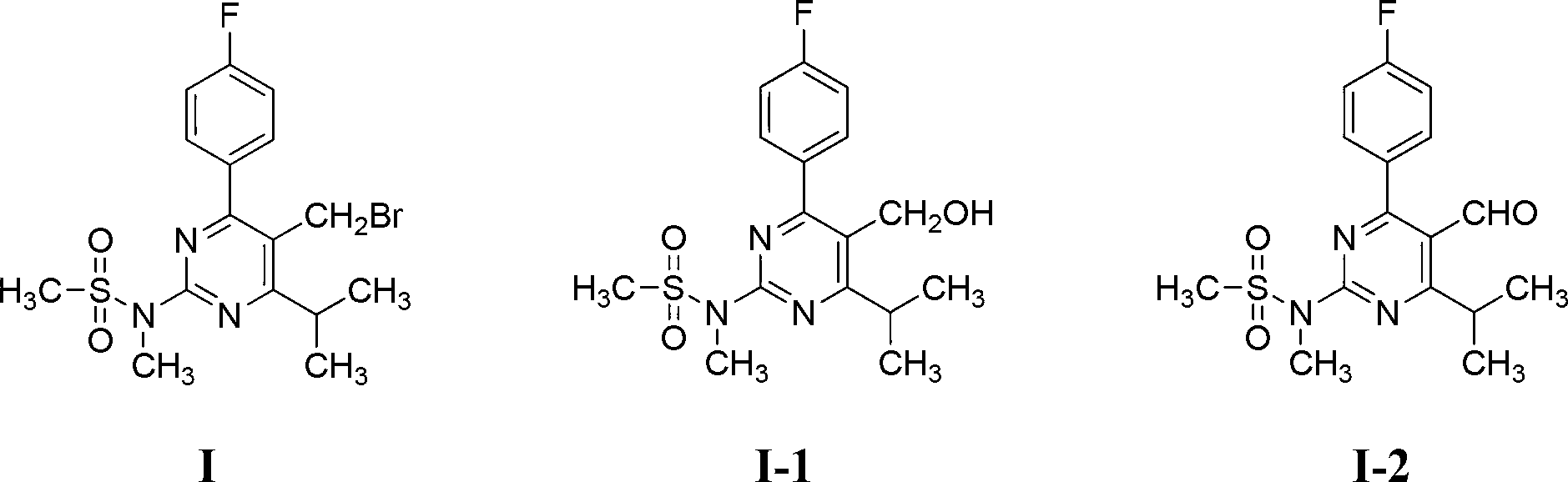
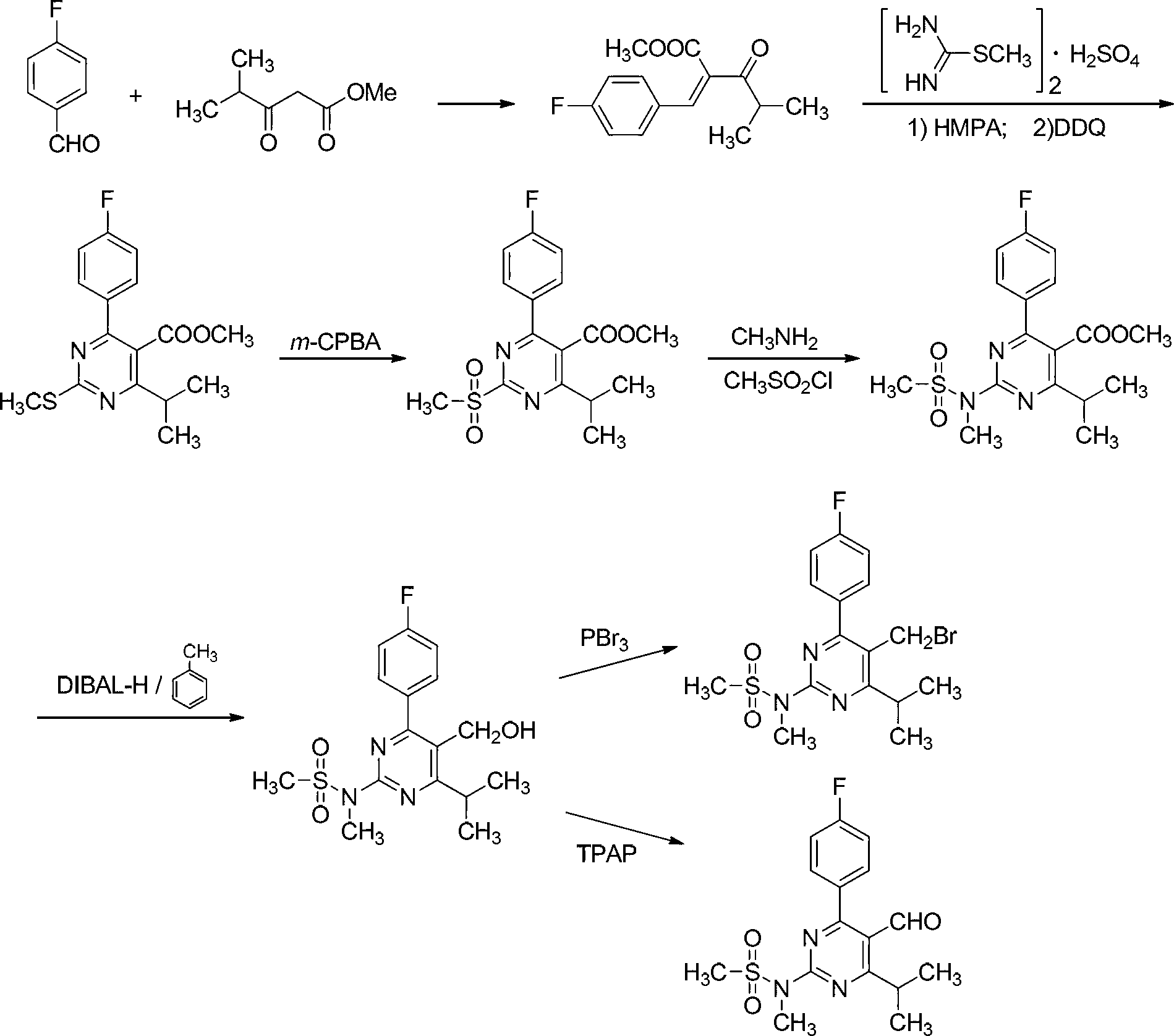
Method for preparing rosuvastatin calcium intermediate containing brooethyl, hydroxymethyl or formyl
A technology for the reaction of methylguanidine hydrochloride and methanesulfonyl chloride, which is applied in the field of medicine and chemical industry, can solve the problems of high toxicity, high production cost, and high price, and achieve the effects of mild reaction conditions, high yield, and cheap reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: Synthesis of 1-(4-fluorophenyl)-4-methylpentane-1,3-dione (Compound V)
[0034]
[0035]Under nitrogen protection, add 12.18g (0.3mol) of sodium hydride and 150mL of 1,4-dioxane into a 500mL three-necked flask, and add dropwise 13.81g (0.1mol) of p-fluoroacetophenone and isobutyl A solution of 11.62g (0.1mol) of ethyl acetate dissolved in 100mL of 1,4-dioxane. The reaction solution was stirred at 85°C for 6h, then cooled to room temperature, poured into 400mL glacial hydrochloric acid (2M), and stirred. Extract with 250 mL of ethyl acetate, wash with 100 mL of water and 100 mL of brine in turn, and dry over anhydrous magnesium sulfate. Filtrate, the filtrate is concentrated, and finally obtain liquid V through vacuum distillation, productive rate 85%, nuclear magnetic data ( 1 HNMR, 500MHz, internal standard TMS, solvent CDCl 3 ) as follows: δppm 7.93-7.87 (m, 2H, Ar-H), 7.18-7.12 (m, 2H, Ar-H), 4.15 (s, 1H, CH 2 ),2.61(m,1H,CH of i Pr),1.32(d,J=7.0Hz...
Embodiment 2
[0036] Example 2: Synthesis of 1-(4-fluorophenyl)-2,4-dimethylpentane-1,3-dione (compound IV)
[0037]
[0038] Under the protection of nitrogen, 6.25g (30mmol) of compound (V), 4.14g (30mmol) of potassium carbonate, 2.85mL of methyl iodide (45mmol) and 15mL of acetone were successively added into a 100mL three-necked flask, and the reaction was stirred at room temperature for 48h. Add 10 mL of n-heptane to the reaction solution, filter out the solid, and wash with 20 mL of acetone / n-heptane (1:1). The organic layers were combined, the solvent was evaporated under reduced pressure, and the residue was dissolved in 10 mL of ethyl acetate. The solution was washed successively with 12mL hydrochloric acid (2M), 10mL saturated sodium bicarbonate solution, 20mL water and 10mL brine, and dried over anhydrous magnesium sulfate. Filtration, decompression evaporates solvent and obtains white solid IV, productive rate 96%, fusing point is 44-45 ℃, nuclear magnetic data ( 1 HNMR, 500...
Embodiment 3
[0039] Example 3: Synthesis of 4-(4-fluorophenyl)-6-isopropyl-N,5-dimethylpyrimidin-2-amine (compound III)
[0040]
[0041] A solution of 2.00 g (9.0 mmol) of compound (IV), 0.98 g (9.0 mmol) of methylguanidine hydrochloride and 5.86 g (18.0 mmol) of cesium carbonate dissolved in 20 mL of 2-methyltetrahydrofuran was sequentially added to a 100 mL three-necked flask. The mixture was stirred at 70°C for 24h. After cooling to room temperature, 20 mL of water was added, and the layers were separated. The aqueous layer was extracted with 20 mL of 2-methyltetrahydrofuran, washed with 20 mL of brine, and dried over anhydrous magnesium sulfate. The solvent was distilled off under reduced pressure, the residue was washed with 40 mL of water / methanol (1:1), filtered, and dried to give yellow solid III with a yield of 93% and a melting point of 140-141°C. The NMR data ( 1 HNMR, 500MHz, internal standard TMS, solvent CDCl 3 ) as follows: δppm 7.52-7.47 (m, 2H, Ar-H), 7.16-7.10 (m, 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com