Use of combined serum metabolic marker in preparation of kit for diagnosing progress of hepatopathy, kit, and method using kit to screen serum metabolic markers
A technology for metabolic markers and screening methods, which is applied in the field of combined serum metabolic markers in the preparation of kits for diagnosing the development of liver diseases, can solve problems such as difficulties in monitoring the occurrence and development of liver diseases, and achieves alleviation of difficulties in monitoring the occurrence and development of liver diseases. The effect of application value and fast screening speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] (1) Instruments and materials:
[0078] American AB Sciex 5500 tandem mass spectrometer;
[0079] Angilent 1290 liquid chromatograph;
[0080] Agilent C18 column (2.1 x 50 mm, 1.7 μm).
[0081] (2) Drugs and reagents:
[0082] Standard substances: GCDCS, GCDCA, GDCA, GCA, TCDCA were purchased from Sigma;
[0083] The isotope internal standards of the above bile acids are sodium glycochenodeoxycholate sodium sulfate-d4 (GCDCS-d4), glycocholic acid-d4 (Glycocholic acid-d4, GCA-d4), taurochenodeoxycholic acid-d4 ( TCDCA-d4) were all purchased from American Cerilliant Company;
[0084] Formic acid: chromatographically pure (MERCK company);
[0085] Acetonitrile: chromatographically pure (MERCK company);
[0086] Ammonium bicarbonate: analytically pure purchased from Sinopharm Group (Shanghai);
[0087] Double distilled water: Thermo.
[0088] (3) Detection solution
[0089] Stock solutions of reference substances: GCDCS, GCDCA, GDCA, GCA and TCDCA were prepared int...
experiment example 1
[0115] In order to show that the method of the present invention for screening combined serum metabolic markers with the kit for diagnosing the development of liver disease has the advantages of fast screening speed, low screening cost, and the advantages of batch screening, and can well screen the combined serum in patient serum. The screening of metabolic markers effectively alleviates the difficulty in monitoring the occurrence and development of liver diseases in the existing clinical treatment of liver diseases.
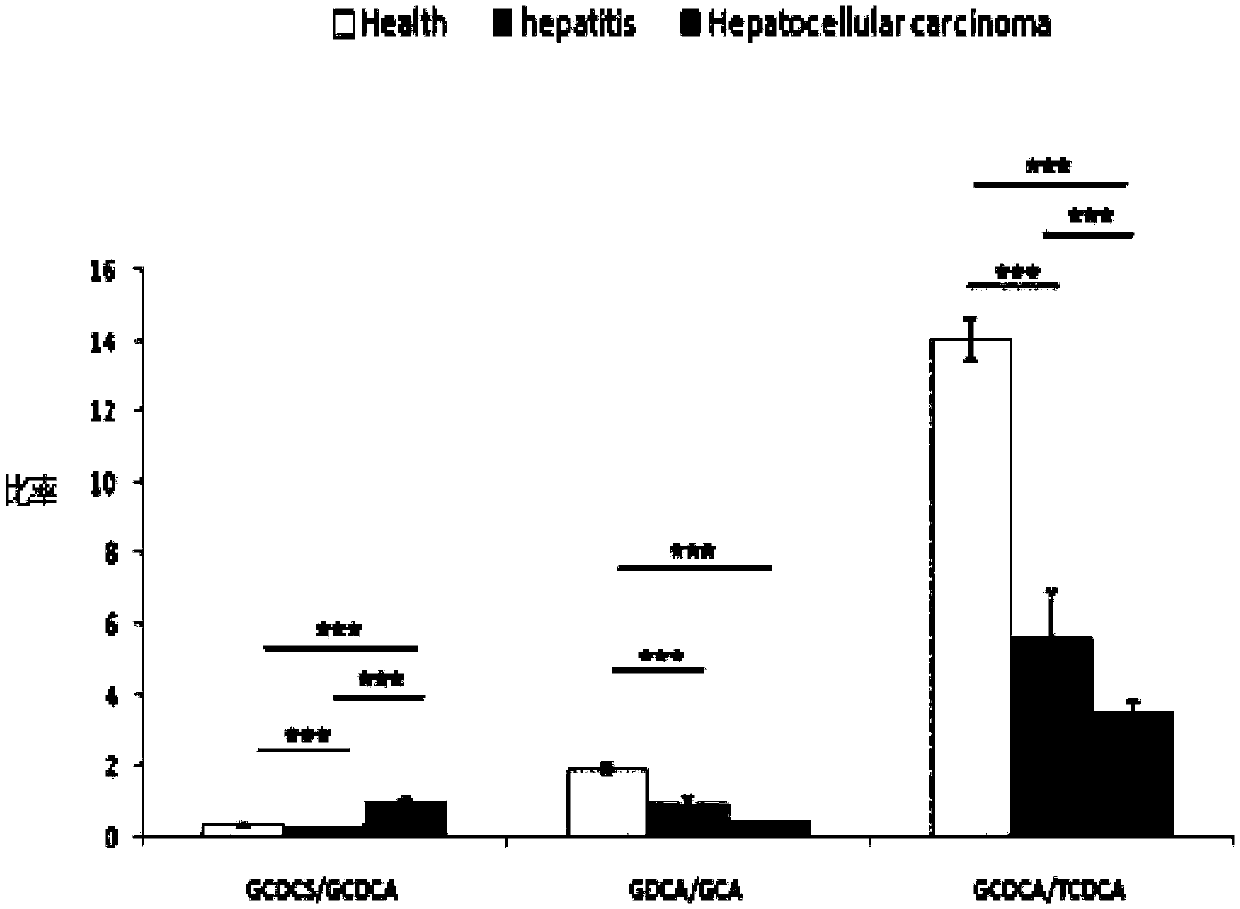
[0116] Now select 374 cases of serum samples, including 234 cases of normal samples, 67 cases of acute hepatitis samples, and 73 cases of liver cancer samples. Use the screening method of the present invention to screen the combined serum metabolic markers in each serum sample. The screening method With embodiment 1.
[0117] The content of GCDCS, GCDCA, GDCA, GCA and TCDCA in the above-mentioned serum samples in the normal control group (Health), acute hepatiti...
experiment example 2
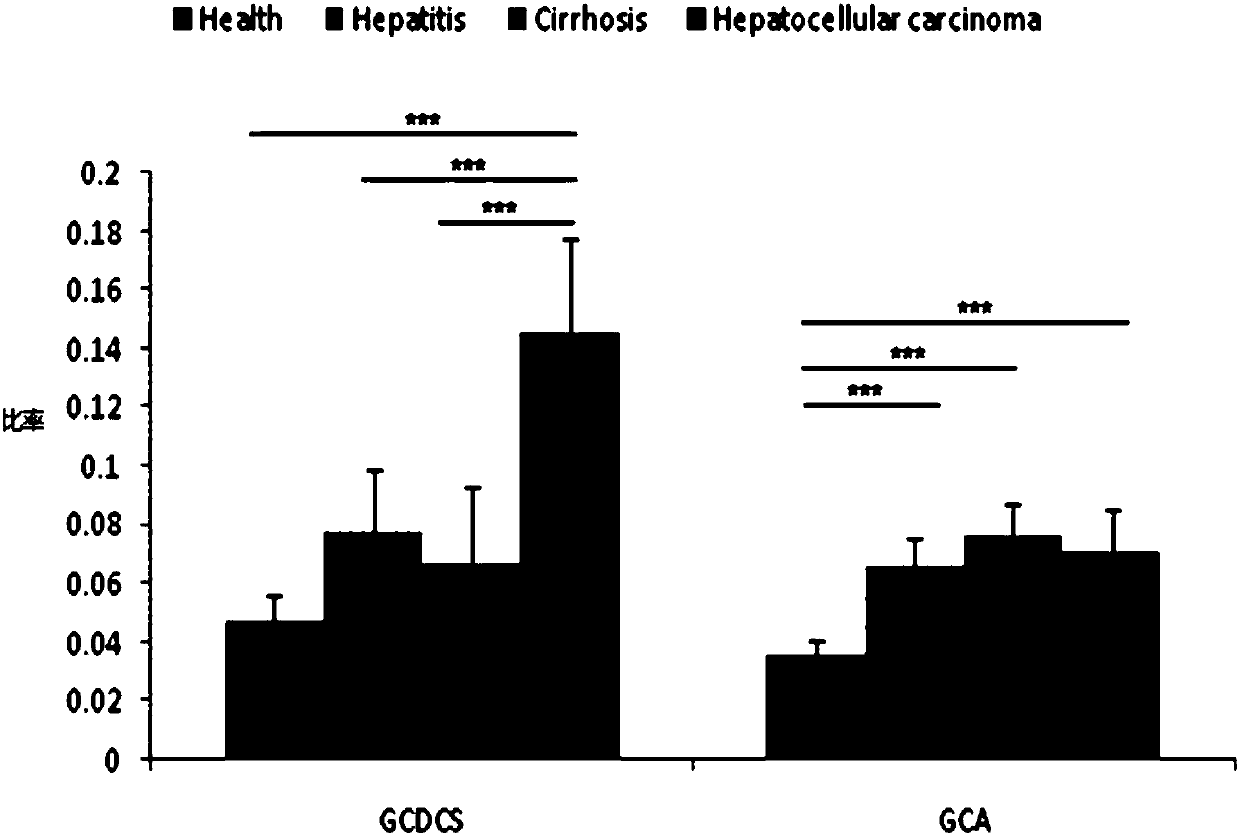
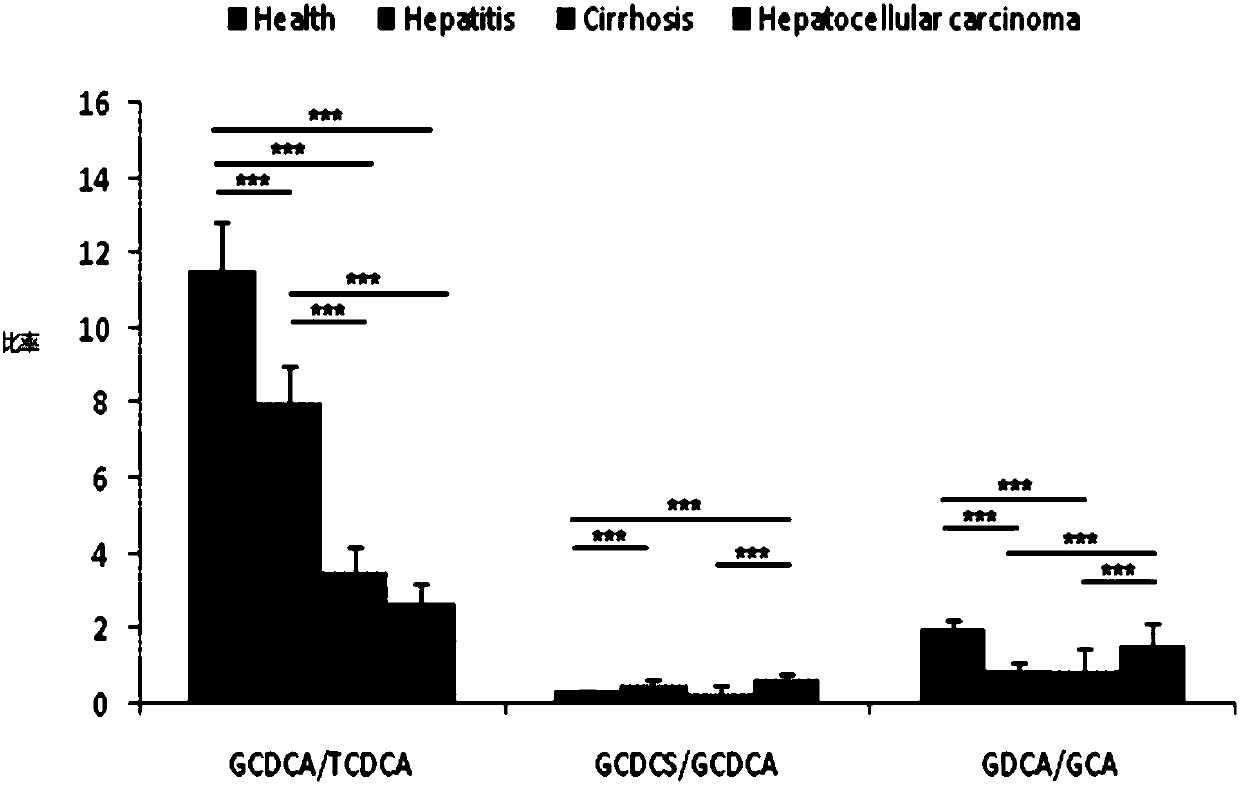
[0121] In order to show that the method of the present invention for screening combined serum metabolic markers with the kit for diagnosing the development of liver disease can well screen the combined serum metabolic markers in patient serum, thereby effectively alleviating the current problems in the clinical treatment of liver diseases. The development and monitoring of liver disease is difficult.
[0122] 135 samples are specially selected, including 45 normal samples, 42 chronic hepatitis samples, 25 liver cirrhosis samples and 23 liver cancer samples, corresponding to normal control group (Health), hepatitis group (hepatitis), liver cirrhosis group (Cirrhosis) and liver cancer group (Hepatocellular caicinoma), using the screening method of the present invention to screen the combined serum metabolic markers in the serum samples of each of the above groups, the screening method is the same as in Example 1. At the same time, the total bile acid content in each serum sample...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com