Glycocholic acid detection kit
A technology for detecting kits and glycocholic acid, applied in measuring devices, color/spectral characteristic measurement, instruments, etc., can solve the problems of poor reagent stability, cumbersome operation, low sensitivity, etc., and achieve the effect of low cost and simple preparation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0083] Screening experiment of specific component concentration and formula ratio in the present invention
[0084] 1. Concentration screening experiment of 8-aniline-1-naphthalenesulfonic acid
[0085] 1.1 Experimental method:
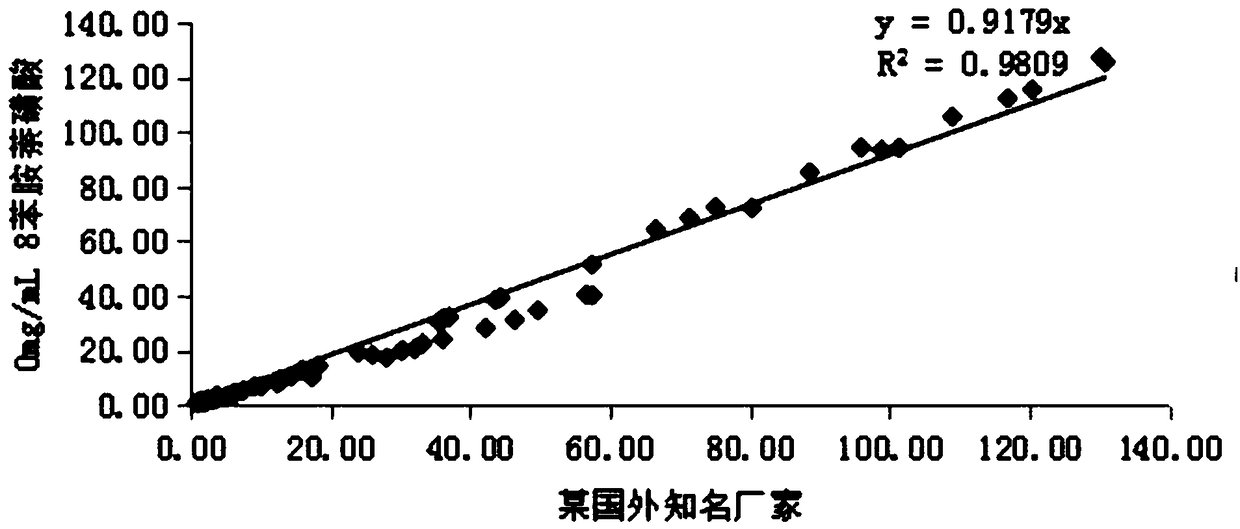
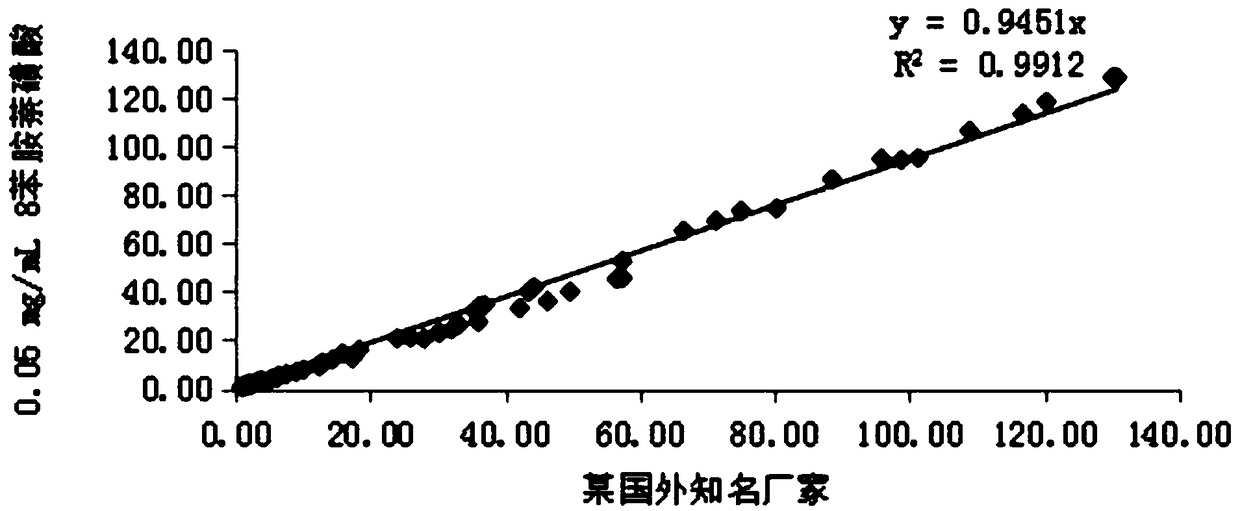
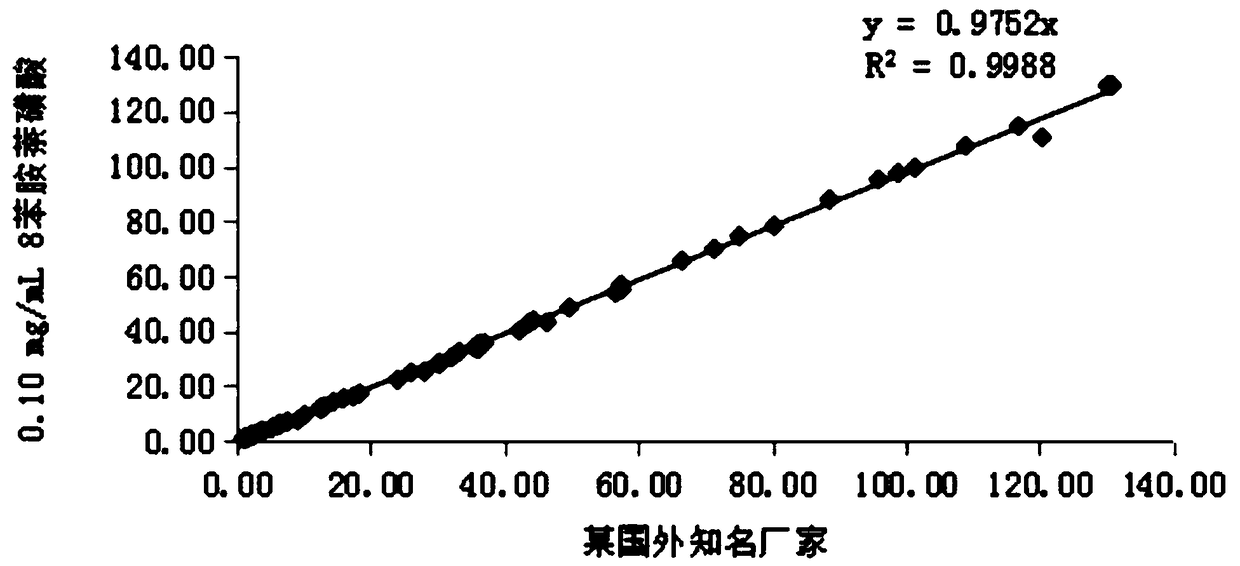
[0086] The following experiment was designed to investigate the effect of 8-aniline-1-naphthalenesulfonic acid in glycocholic acid (latex enhanced immune turbidimetric method) kit. Add 8-aniline-1-naphthalenesulfonic acid to the R1 solution, and finally prepare to contain 8-aniline-1-naphthalenesulfonic acid with final concentrations of 0mg / mL, 0.05mg / mL, 0.10mg / mL, 0.15mg / mL , 0.20mg / mL, 0.25mg / mL, 0.30mg / mL solutions. The above seven groups of solutions were used as the R1 reagent in the self-made CG (latex enhanced immunoturbidimetric method) reagents, matched with the self-made R2 reagents and calibration quality control products, using the two-point endpoint method as the analysis method, and setting the rising reaction as the reaction direction. SPL...
Embodiment 2
[0127] A glycocholic acid kit, including the following components:
[0128] Glycocholic acid R1 reagent:
[0129]
[0130] Glycocholic acid R2 reagent:
[0131]
[0132] The preparation method of glycocholic acid-protein conjugate in this kit includes the following steps:
[0133] 1) Weigh the pure glycocholic acid and dissolve it with DMF to prepare a 10 mg / mL glycocholic acid solution; use DMF to prepare 8 mg / mL NHS solution; use DMF to prepare 10 mg / mL EDC solution; use ultrapure water to prepare 10 mg / mL cattle Serum albumin solution;
[0134] 2) Take 45ul and 33ul of NHS solution and EDC solution respectively, add them to 70ul glycocholic acid solution and stir for 30min at room temperature;
[0135] 3) Add the mixed solution obtained in step 2) to 0.5ml bovine serum albumin solution and stir for 12h at room temperature;
[0136] 4) Place the mixture obtained in step 3) in 0.01 mol / L citrate buffer at pH 6.0, and dialyze for 12 hours to obtain glycocholic acid-protein conjugate.
[0...
Embodiment 3
[0143] Glycocholic acid R1 reagent:
[0144]
[0145]
[0146] Glycocholic acid R2 reagent:
[0147]
[0148] The preparation method of glycocholic acid-protein conjugate in this kit includes the following steps:
[0149] 1) Weigh pure glycocholic acid, dissolve it with DMF to prepare 30mg / mL glycocholic acid solution; prepare 15mg / mL NHS solution with DMF; prepare 40mg / mL EDC solution with DMF; prepare 40mg / mL cattle with ultrapure water Serum albumin solution;
[0150] 2) Take 40.8ul and 30ul of NHS solution and EDC solution respectively, add them to 63.6ul glycocholic acid solution, and stir for 30min at room temperature;
[0151] 3) Add the mixed solution obtained in step 2) to 0.9ml bovine serum albumin solution, and stir for 12h at room temperature;
[0152] 4) Place the mixture obtained in step 3) in 0.01 mol / L citrate buffer at pH 6.0, and dialyze for 12 hours to obtain glycocholic acid-protein conjugate.
[0153] The preparation method of anti-glycolic acid antibody coated latex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com